Does the Valence Electron Arrangement of Tungsten Follow Hund's Rule?
- Details
- Category: Tungsten Information
- Published on Sunday, 10 September 2023 23:32
The valence electron arrangement of tungsten does not follow hund's rules. Tungsten is one of the transition metal elements in the sixth period (second long period) of the periodic table of elements VIB group. Transition metal elements are obviously different from other elements because of their unfilled valence layer d orbitals based on the eighteen-electron rule.
Macroscopically, the melting points of transition metals are high and regular: the melting points of transition metals from left to right and from top to bottom in the periodic table of elements tend to be located at the melting point of tungsten in the lower right corner (3422 ℃), and they are hard enough to react with halogen gas and form halogen tungsten cycle, which is the most raw material for manufacturing filaments.
Compared with the main group elements, the valence electron arrangement of transition metal elements such as tungsten does not follow the law of hund's rules. Hund's rules is a simple approximate description of the phenomenon of energy level staggering. The valence electrons of the elements before the fifth period basically conform to this rule, but the valence electrons of the elements after the fifth period often do not conform to this rule, especially the lanthanide and actinide elements almost do not conform to this rule. The electron configuration of the valence layer of transition elements is characterized by that the electrons in the d orbital are in an unfilled state (except for Pd), and the outermost layer has only 1~2 electrons. Their valence electron configuration is (n-1)d1-9ns1-2 (Pd is 4d10).
In order to understand the interlacing of energy levels in complex cases, we can generally refer to F A Cotton's diagram of orbital energy changing with atomic number in 1962 besides hund's rules. The atomic number of tungsten is 74. It can be seen in the figure that the energy of 6s and 5d is almost the same near the atomic number 74.
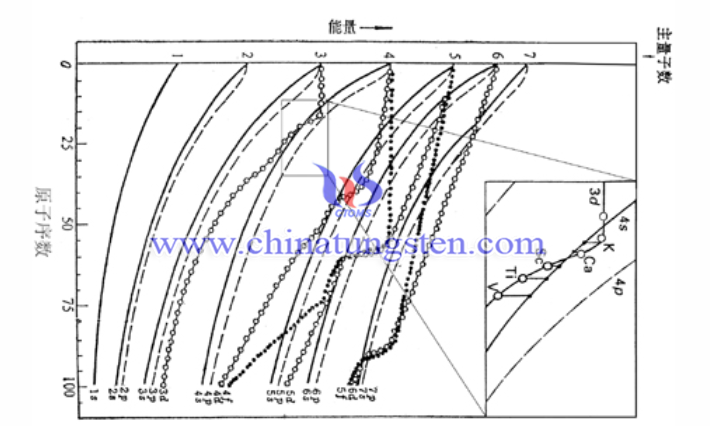
According to the convention of the same family of chromium and molybdenum, the half-filled 5d56s1 configuration should be arranged, but the relativistic effect makes the 6s orbital energy shrink centripetal, so it is more inclined to adopt the 5d46s2 configuration.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com