Light Absorption and Photocatalytic Characteristics of Tungsten Disulfide Nanomaterials
- Details
- Category: Tungsten Information
- Published on Tuesday, 23 August 2022 20:35
Compared with semiconducting materials, tungsten disulfide nanomaterials exhibit higher light absorption, and photocatalytic properties are another important property. For semiconductor materials, light absorption properties are very important, especially for photocatalysis. When WS2 absorbs photons, transitions between in-band, out-of-band, and impurity defects occur, which can form specific absorption spectra. The characteristic absorption peak of bulk WS2 is near the wavelength of 910 nm and is located in the near-infrared (NIR) region. By forming nanostructures, a blue shift of the WS2 characteristic absorption peak can be observed.
A researcher tested WS2 nanosheets with an ultraviolet-visible (UV-Vis) spectrometer and obtained its UV-Vis absorption spectrum. The synthesized WS2 nanosheets have a distinct absorption peak in the wavelength range of 380-530 nm and a certain absorption peak in the wavelength range of 600-700 nm. In addition, WS2 with nanostructures exhibited higher light absorption compared to the low absorption of the semiconducting materials.
For example, other researchers have prepared ultrathin WS2 nanoribbons (N-WS2), and this prepared nanomaterial shows strong and enhanced optical absorbance as the wavelength increases from 200 to 1000 nm.
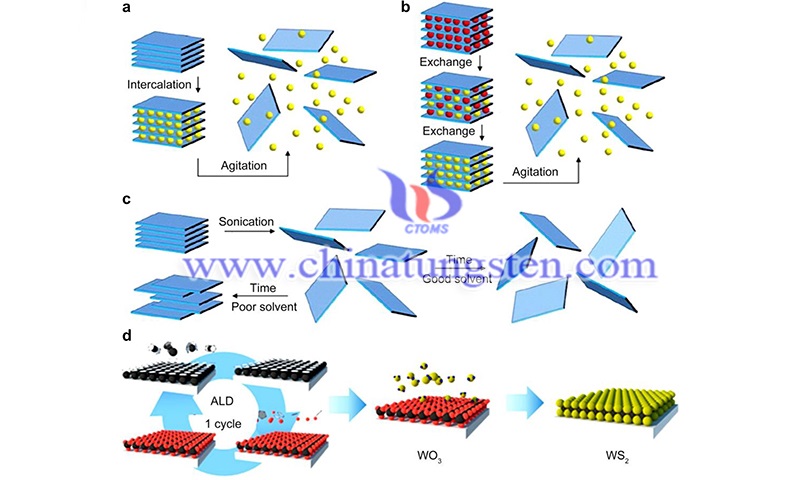
Thus, the relatively broad absorption range in the visible region coupled with the strong absorption suggests that tungsten disulfide nanomaterials are promising for a wide range of photocatalytic applications, such as the degradation of organic dye molecules and the decomposition of water to produce hydrogen gas in the UV-NIR light.
Photocatalytic properties are another important property of WS2 which can be governed by the electronic band structure. In order to realize the wide application of WS2 in photocatalysis, it is helpful to understand its basic photocatalysis properties. In general, the band structure of semiconductor materials usually contains a valence band filled with electrons and an empty conduction band with a band gap between them. When exposed to photons with energies greater than the band gap energy, the electrons of the semiconductor can be transferred from the valence band to the conduction band, while leaving holes in it.
The specific process consists of three steps: first, in sunlight, the semiconductor absorbs photon energy and is excited to produce photogenerated electron-hole pairs; then, photogenerated charges are separated and migrate on the catalyst surface; and finally, a series of redox reactions occur between the photogenerated charges and organic pollutants or H2O. Because WS2 has a proper band structure, it can be one of the promising candidates for driving redox reactions to photodegrade organic pollutants. Encouragingly, the researchers developed WS2 nanosheets with a thickness of 100 nm that exhibit strong UV and visible light photocatalysis activity, indicating a novel and promising broad solar spectrum photocatalyst.
The behavior of light-generated charge carriers was investigated by performing photoelectrochemical (PEC) analysis to reveal the photoelectric interactions in WS2 nanosheets. The results confirmed that both UV and visible light excitation induce electron-hole pairs in WS2 nanosheets, which are essential for the photocatalytic process. In addition, the researchers recorded the open circuit potential (OCP) response of the WS2 anode to near-infrared light. it took about 250 s for the WS2 nanosheet anode to achieve a larger potential, indicating the relatively long lifetime of the light-induced charge carriers in the WS2 nanosheets. This could reduce carrier recombination and facilitate carrier participation in the photocatalytic reaction process.
Thermodynamically, water is a stable compound. Under standard conditions, 1 mol of water is decomposed into hydrogen and oxygen, requiring additional energy of 237 kJ. As mentioned earlier, the VBM and CBM energies of bulk WS2 do not match the redox potential for water oxidation and reduction, although the band gap can be adjusted by stripping and forming nanostructures. Therefore, the efficiency of using a single WS2 as a photocatalyst to split water is not high. Currently, a feasible strategy for WS2 application in photocatalytic water decomposition is to combine WS2 with other photocatalysts to form heterogeneous structures.
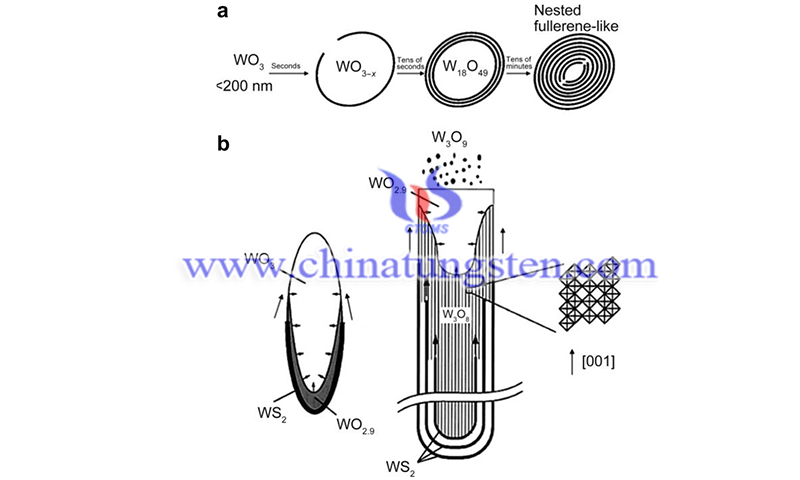
However, its rate characteristics are low due to the lack of active sites for H2 evolution. When WS2 is introduced as a co-catalyst, photogenerated electrons are transferred to WS2 particles, which can induce the hydrogen evolution process due to the good catalytic ability of WS2 for the reduction of H+. However, the recombination of photogenerated carriers limits the application of WS2 in photocatalysis, which is one of the immediate concerns in future research.
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. Tungsten disulfide nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com