Enhanced Photoelectrochemical Performance of WO3 Film Doped with HfO2 Passivation Layer
- Details
- Category: Tungsten Information
- Published on Sunday, 22 August 2021 17:39
With ever-increasing demands of energy and environmental issues caused by extensive combustion of traditional fossil fuels, the quest for clean and sustainable energy sources as alternatives has attracted extensive research interest. Hydrogen seems to be a promising alternate due to its nontoxicity, ideal combustion efficiency and high gravimetric energy density. Among many accepted ways of hydrogen generation, photoelectrochemical (PEC) water splitting has been widely regarded as an appealing solution.
Tungsten trioxide (WO3) is considered as the most critical semiconductor material having promising PEC water splitting capabilities due to its small bandgap (of 2.5–2.8 eV) and hole diffusion length of (~150 nm). Being an n-type semiconductor, WO3 possesses high electron hall mobility (12 cm2V−1s−1) with the absorption of 12 % of the solar spectrum.

Doping passivation layer on the surface of semiconductor could enhance the photocatlytic performance of WO3 because the passivation layer can affect surface defect sites to inhibit the electron-hole recombination on the surface of semiconductor. Hafnium oxide (HfO2) is a wide band gap material, which is widely used in the dye-sensitised solar cell and metal-oxide-semiconductor high-electron-mobility transistor (MOS-HEMTs) as a passivation layer.
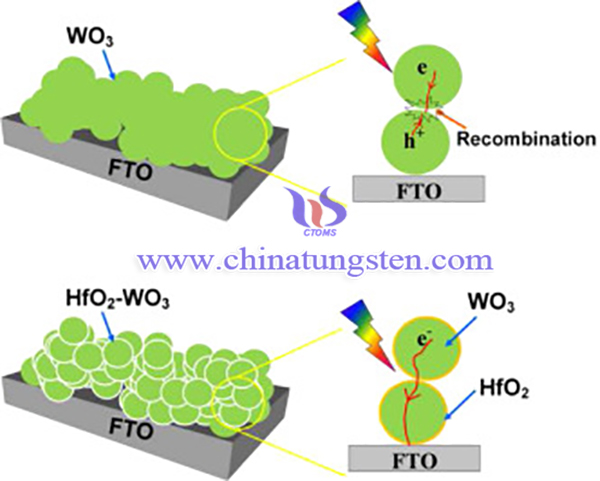
HfO2 passivation layer is reported to be doped on the surface of WO3 films by a simple solvothermal method to obtain an enhanced photoelectrochemical performance. The fabrication method of it is as following steps:
Firstly, WO3 nanoparticles were fabricated by a solution method. In a typical experiment, Polyvinyl Pyrrolidone (PVP K-30, 2.0 g) was suspended in deionized (DI) water (15.0 mL). Ammonium metatungstate ((NH4)6H2W12O40·xH2O, 1.478 g, Aldrich) was dissolved in 10.0 mL distilled water and then dropped into the PVP solution under stirring. After ultrasonic treating for 30 min, the solution was stirring for 2 h. Then, 2.0 g polyethylene glycol (PEG1000) was added, and the system was stirred for additional 4 h. The sol-gel was dried at 80 °C, and sintering at 600 °C for 1 h in air.
Secondly, 0.3 g of as-prepared WO3 nanoparticles were suspended in 30 mL concentrated sulfuric acid solution containing 14 mg HfO2. After solvothermal treatment at 110 °C for 2 h, the product was centrifuged,the product was washed with deionized water and absolute ethanol. Then the sample was gained after dried in an oven at 60 °C. For comparison, bare WO3 nanoparticles were also treated in this way without the addition of HfO2.
A nanoporous HfO2-WO3 film-coated FTO electrode was prepared by using a doctor-blade technique. Before coating, the FTO substances were cleaned up in acetone/isopropanol/methanol/DI water, respectively. 0.175 g HfO2-WO3, 0.05 g PEG20000, 0.025 g ethylcellulose, 0.8 mL terpineol, 0.2 mL acetylacetone and 0.2 mL triton were added to an agate jar. The paste was acquired by ball milling for 8 h. The resulting paste was squeezed over the FTO substrate by a doctor-blade coater and dried at 60 °C for 30 min. Finally, it was calcined at 450 °C for 30 min with a heating rate of 2 °C/min to obtain the HfO2-WO3 film electrode. For comparison, WO3 film electrode was also fabricated.
In summary, HfO2 passivation layer (Hafnium oxide) is reported to be doped on the surface of WO3 films by a simple solvothermal method to obtain an enhanced photoelectrochemical performance. The morphology and size of the samples have not been changed obviously. HfO2-WO3 film electrode showed the enhanced photoelectrochemical activity under visible light irradiation in comparison with bare WO3 film electrode.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com