A New Solid–Gas Phase Reaction to Prepare Ammonium Paratungstate(APT)
- Details
- Category: Tungsten Information
- Published on Sunday, 03 May 2020 13:31
The ammonium paratungstate (APT) is the most important starting material in the tungsten industry, where usually its most stable APTÂ4H2O form is used. Tungsten compounds such as, tungsten oxides, tungsten carbides or tungsten metal can be prepared with APT. All these tungsten products have important applications in a many fields. Among which, tungsten oxides are widely used in catalysts, gas sensors, and photocatalysts. Tungsten carbide products have been used as catalysts as well, but another important application field they shared is being the hard component in cutting and drilling tools.
In the industry, the usual synthesis method to produce APT by the wet chemical process. The process are as follows: the first step is the alkaline digestion, when the tungsten content is extracted from the tungsten ore concentrates, scrap metal or oxides. The tungsten solution is obtained. In the second step, impurities such as silica, phosphate and fluoride ions are removed by adding aluminum sulfate or magnesium sulfate to the solution.]. The third step is the ion exchange, when the sodium ions are replaced by ammonium ions. First an organic solvent is added with phase transfer catalyst to the aqueous solution, so that the tungstate content is moved to the organic phase. After that aqueous ammonia solution is added to the separated organic phase. At the end of this step an aqueous solution of ammonium isopolytungstate is obtained, from which the APT is produced by crystallization.
Although wet chemical process is a well-developed method, it has its own shortages. For example, it has a higher consumption in energy and chemicals. Thus, this method does not meet the concept of green chemistry. What’s more, it is very sensitive to the reaction.
In order to conquer these disadvantages, a new solid–gas phase reaction to prepare ammonium paratungstate was developed to meet the needs. The detailed procedures follows:
The reactions between the precursor tungsten oxide powders and the NH3 and H2O vapors were carried out at room temperature in a sealed plastic box. WO3 powder and aqueous ammonia solution were placed separately into the box, where the reagents were able to react only via the gas phase. The partial pressures of ammonia and water vapor were controlled by varying the concentration of ammonia in the solutions. The equilibrium partial pressure of NH3 and H2O vapors values were calculated from the concentrations of the aqueous solutions.
In each reaction 500 mg WO3 powder and 25 ml aqueous ammonia solution were used. The yield of the reactions was calculated from the mass difference before and after the reaction.
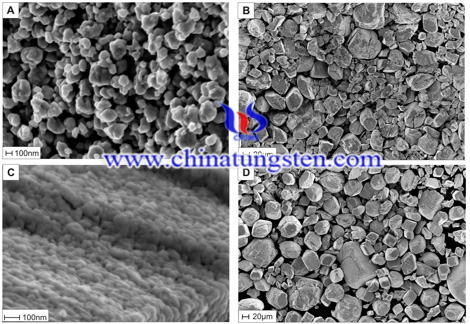
Then the as-prepared APT was characterized thoroughly, by powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and thermal analysis (TG/DTA-MS).
The as-prepared APT was characterized with powder XRD, FTIR, Raman spectroscopy and TG/DTA-MS measurements. These measurements proved that the as-prepared APT is equal to the commercial one. SEM and TEM images revealed that nanosize (40-300 nm) APT was produced for the first time, which can be important in research and industry due to its greater surface area.
The results showed that this novel synthesis of APT is not sensitive to the reaction conditions, in contrast to the previously only available wet chemical crystallization process. According to the measurements, the asproduced APT is equivalent to the commercial ones. As an additional feature, our method is capable to yield APT nanoparticles for the first time, which is important for both research and industry due to the greater surface area.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com