Heterostructured Tungsten Trioxide Catalyst
- Details
- Category: Tungsten Information
- Published on Sunday, 04 August 2019 20:23
Since the first discovery of photocatalysis by Japanese scientists in 1972, photocatalysis has been applied in many fields, including water treatment, and its development prospects have been widely recognized. Tungsten trioxide is regarded as one of the most promising photocatalysts because of its visible light response. However, one of the difficult problems it faces is its poor catalytic activity. Therefore, it is still the focus of photocatalytic research to find a simple synthesis method, low cost and high photocatalytic activity of tungsten trioxide.
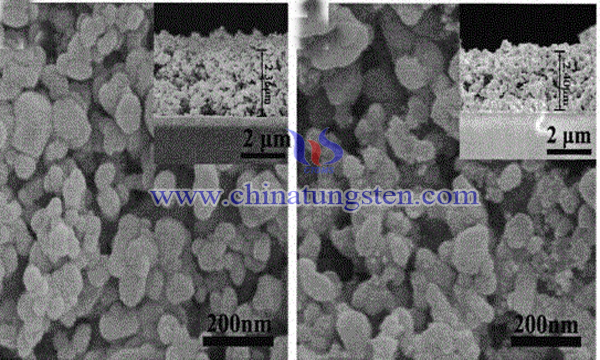
In principle, the transverse heterostructure of tungsten trioxide is the continuous growth of the second material at the edge of the existing region of the first material. The transverse heterojunction structure of tungsten oxide can be fabricated by in situ adjusting the gas phase reactants during the growth of tungsten oxide two-dimensional crystals. In order to achieve the above purpose, some scholars have developed a six-petal flower-like tungsten trioxide photocatalyst with heterostructure. The catalyst consists of monoclinic WO3 and orthorhombic WO3·0.333H2O, and its chemical formula is WO3/WO3·0.333H2O.
The preparation method is a two-step hydrothermal method: the first step uses pure water as a solvent, and then ammonium tungstate, ammonium fluoride and concentrated nitric acid are sequentially added under stirring, and the hydrothermal reaction is carried out, followed by centrifugal washing and drying to obtain a fluorinated tungsten trioxide; Add 20mL of water to the beaker under temperature, add 5mmol of ammonium tungstate and 5mmol of ammonia fluoride under stirring, add 5ml of concentrated nitric acid, stir for 30min, add the mixed solution to the PTFE lining, 180°C The mixture was hydrothermally reacted for 24 hours at a constant temperature, and dried by centrifugal washing to obtain a fluorinated tungsten trioxide.
In the second step, the fluorinated tungsten trioxide obtained in the first step is mixed with an aqueous hydrogen peroxide solution and then hydrothermally reacted, then centrifuged and dried, and the fluorinated tungsten trioxide obtained in the previous step is placed in 5 mL of pure water and 10 mL of 30%. The mixture of the hydrogen peroxide solution was stirred for 30 minutes, and the mixed solution was added to the PTFE lining, and the mixture was hydrothermally reacted at 180 °C for 24 hours, and dried by centrifugation.
Weigh 0.1g of a heterogeneous structure tungsten trioxide catalyst sample of six petals, and add 200ml of RhB aqueous solution, wherein the concentration of RhB is 10mg/L, firstly stirred for 30min in the dark, so that the dye reaches the adsorption/desorption equilibrium on the catalyst surface. Then, the xenon lamp source is turned on for photocatalytic reaction under visible light irradiation, and the supernatant is detected by a spectrophotometer. After 5 hours of illumination, the hexagonal flower-like tungsten trioxide catalyst containing heterostructure degraded RhB by up to 80%. It can be seen that the hexagonal flower-like tungsten trioxide catalyst containing heterostructure has high catalytic activity for RhB. And the production method is simple, the cost is low, the reproducibility and the stability are good.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com