Mechanism of Solid Phase Dissolution and Reprecipitation
- Details
- Category: Tungsten Information
- Published on Wednesday, 28 February 2018 14:23
After the liquid phase formation and particle rearrangement, the densification process of the sintered body has made great progress during the sintering. However, the resistance of the liquid viscous flow is increased because the particles are formed to a certain extent and form a bridge. The phase of particle rearrangement cannot be completely compact, so it is necessary to further advance through solid phase dissolution and reprecipitation.
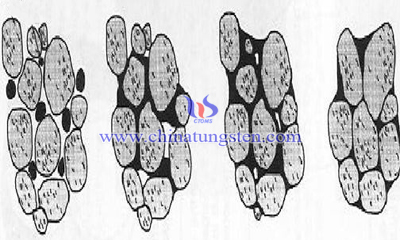
The mechanism of solid phase dissolution and reprecipitation is mainly dependent on the difference of chemical positions in different regions (saturated equilibrium concentration difference), resulting in liquid phase migration between particles or different parts of particles.
Because of the different size of the particles, the irregular shape of the surface and the different curvature of each part, the saturation solubility is not equal, that is, the degree is not equal. When the substance between particles or between different parts of a particle migrate between the liquid phase, the parts of small particles or particles with large surface curvature (at the point of the particle) dissolve more.
On the contrary, the dissolved matter precipitates on the surface of a large particle or with a negative curvature. Like the calculation of the saturated vapor pressure, the particle with the radius of curvature r is the difference of the equilibrium concentration difference between the saturated concentration difference and the plane (r infinity):
ΔN=2γslδ3 /(kTrNp)(1)
Where,
γsl-- Surface tension of solid-liquid
δ-- Lattice constant,
k-- Boltzmann constant,
T-- Temperature,
Np--Plane concentration.
Therefore, the small particles precede large particles dissolving. The dissolution and reprecipitation process makes the particle shape gradually become spherical, where the small particles decrease or disappear, and large particles grow up. At the same time, the smooth surface and spheroidization of solid particles reduce the rearrangement resistance of particles. Particles rely on shape adaptation to achieve closer packing and accelerate sintering shrinkage.
- Tungsten Carbide Manufacturer & Supplier, Chinatungsten Online: tungsten-carbide.com.cn
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com