Influence Factors of Particle Pattern and Distribution
- Details
- Category: Tungsten Information
- Published on Wednesday, 28 February 2018 14:19
The sintering of tungsten carbide is a liquid phase sintering process. Particle pattern and distribution (mainly refractory carbide particles) will directly affect the properties of the tungsten carbide, such as hardness, strength and so on. Microstructure of liquid phase sintered alloy, namely solid particle shape and distribution, depending on the crystallographic characteristics of solid substances in the liquid wettability or the size of the dihedral angle.
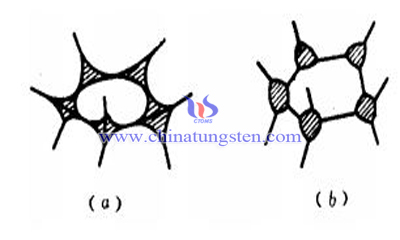
Crystallography of Solid Particles
It mainly refers to the bond type, direction, crystal plane to these three elements. For WC-Co tungsten carbide, WC crystal is combined with covalent and ionic bonds, with high bond strength and less solubility. Because of the non equiax crystal characteristics and the strong bond direction, the WC in the alloy microstructure after sintering remains multilateral.
And for heavy alloy such as tungsten carbide, the metal bond and the ionic bond are mainly in the crystal, of which the bonding strength is less than the cemented carbide, and it is easier to dissolve. Meanwhile, with the weaker bond direction, the liquid phase sintered alloy is recrystallized and grown by dissolving and re precipitation, and the particles after the cooling are mostly oval.
Wettability or Dihedral Angle of Liquid Phase
It will also affect the morphology and distribution of solid particles. According to the theory of fluid solid phase relative wetting, dihedral angle is determined by the ratio of solid-solid interfacial tension γss and liquid interfacial tension γsl:
cos(ψ/2)=1/2*γss/γsl (1)
Where,
ψ-- dihedral angle.
When γsl《γss and ψ<60°, the liquid solid interface spread, complete coverage of solid surface. When the γsl>γss and ψ>120°, the liquid will be isolated droplet distribution in the intersection of the solid phase interface.
When the amount of liquid phase is sufficient to fill all the gaps in the particles and there is no ideal condition for the existence of the pores:
1.ψ=0°,the solidified liquid component forms a continuous film to encircled solid particles.
2.0°<ψ<120°,The liquid phase region is formed between solid particles, which is connected with a number of particles, and the solid particles are not completely separated by the liquid phase.
3.ψ>120°,discrete liquid region is formed and surrounded by solid particles.
- Tungsten Carbide Manufacturer & Supplier, Chinatungsten Online: tungsten-carbide.com.cn
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com