Bismuth Oxide Influencing Tungsten Trioxide Ceramic Property
- Details
- Category: Tungsten Information
- Published on Monday, 15 February 2016 17:16
- Written by qiongyao
- Hits: 235
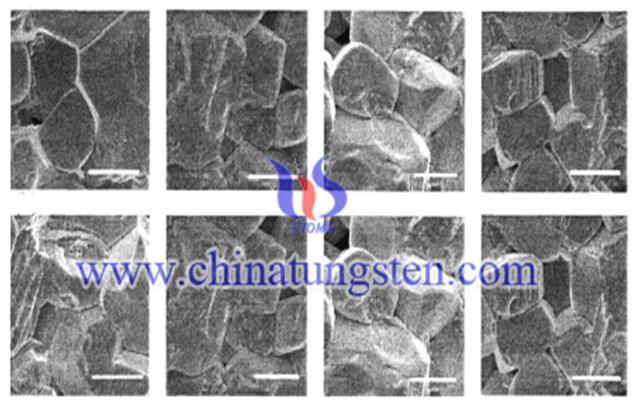 When the doping concentration is 0.2mol% and the sintering temperature is about 1100 ℃, the density of the WO3 ceramics doped Bi2O3 can reach 91%. When the doping concentration is 0.2mol% , WO3 ceramics densification will decline; the sintering temperature is lower than 1100 ℃, the density increases gradually, but when the sintering temperature is 1100 or higher than 1100 ℃, the density of the ceramic will decline ; the best sintering time is 2h, extend the time will make the ceramics sintered density decreases. After analysis, the major causes for this phenomenon are: WO3 melting point is 1273 ℃, Bi2O3 melting point is 860 ℃, the formation of the sublimation are the two substances .
When the doping concentration is 0.2mol% and the sintering temperature is about 1100 ℃, the density of the WO3 ceramics doped Bi2O3 can reach 91%. When the doping concentration is 0.2mol% , WO3 ceramics densification will decline; the sintering temperature is lower than 1100 ℃, the density increases gradually, but when the sintering temperature is 1100 or higher than 1100 ℃, the density of the ceramic will decline ; the best sintering time is 2h, extend the time will make the ceramics sintered density decreases. After analysis, the major causes for this phenomenon are: WO3 melting point is 1273 ℃, Bi2O3 melting point is 860 ℃, the formation of the sublimation are the two substances .
Comparing with non-doped WO3 ceramics, WO3 ceramics doped Bi2O3 varistor voltage declines rapidly, this shows the rapid growth of the ceramic grains causing breakdown voltage drop. When the sintering temperature of WO3 doped Bi2O3 ceramics is 900 ℃, WO3 ceramics do not have pressure-sensitive properties, when the temperature is 900 ℃ ~ 1100 ℃, the pressure-sensitive is maximum coefficient, when the sintering temperature is 1100 ℃, the pressure-sensitive decreases with the temperature rises, it disappears after sintering temperature is higher than 1100 ℃. When Bi2O3 doping concentration is 0.5mol%, the sintering time is 2h.
Doping Bi2O3 having a significant effect on the microstructure of WO3 ceramics; doping can play a role to help promote sintering ceramics; doping can improve the characteristics of the ceramic varistor.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Bismuth Oxide Influencing Tungsten Trioxide Ceramic Grain Growth
- Details
- Category: Tungsten Information
- Published on Monday, 15 February 2016 17:05
- Written by qiongyao
- Hits: 217
 Bismuth trioxide (Bi2O3) is known as yellow powder, it dissolves in water, and it does not dissolve in acid generating bismuth (III) salt. Its melting point is 824 ℃, the boiling point is 1890 ℃. Bismuth trioxide may be obtained from natural bismuth Hua (a mineral), its main source is copper, and bismuth powder can be obtained bismuth trioxide in the air combustion. Bismuth oxide is mainly used in the chemical industry (such as chemical reagents, bismuth salt manufacturing, etc.), glass industry (mainly used for coloring), and the electronics industry (electronic ceramics, etc.). Among them, the electronics industry is the most widely used bismuth oxide industry; it is mainly used in varistors, thermistors, and CRT oxide surge arresters and other fields. In addition, Bi2O3 has high refractive index and dielectric constant, significant fluorescence characteristics and inert to water. Therefore, Bi2O3 is potential decomposition and degradation of water pollutants visible catalyst.
Bismuth trioxide (Bi2O3) is known as yellow powder, it dissolves in water, and it does not dissolve in acid generating bismuth (III) salt. Its melting point is 824 ℃, the boiling point is 1890 ℃. Bismuth trioxide may be obtained from natural bismuth Hua (a mineral), its main source is copper, and bismuth powder can be obtained bismuth trioxide in the air combustion. Bismuth oxide is mainly used in the chemical industry (such as chemical reagents, bismuth salt manufacturing, etc.), glass industry (mainly used for coloring), and the electronics industry (electronic ceramics, etc.). Among them, the electronics industry is the most widely used bismuth oxide industry; it is mainly used in varistors, thermistors, and CRT oxide surge arresters and other fields. In addition, Bi2O3 has high refractive index and dielectric constant, significant fluorescence characteristics and inert to water. Therefore, Bi2O3 is potential decomposition and degradation of water pollutants visible catalyst.
Bi2O3 doping WO3ceramics, when the doping has smaller number, the presence of single phase is WO3; when the doping concentration is greater than 0.2mol%, and it will begin to appear as Bi2WO6 phase. Bi2WO6 phase becomes bigger with the sintering temperature increasing. WO3 phase will disappear in the solid phase reaction sintering process. Bi2O3 can promote the rapid growth of WO3 ceramic grain, compact structure, porosity reduction, and a high concentration of Bi2O3 doped in the grain boundary formation of the second phase Bi2WO6 , which increasing the sintering temperature and time, which can promote the growth of WO3 ceramic grains form more Bi2WO6 phase. According to the analysis results, we can get Bi2O3 doping can be formed during the sintering process in the reaction; the transfer material ultimately provides the energy for the growth of the ceramic grains.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Electrode Liquid-phase Sintering Theory (2/2)
- Details
- Category: Tungsten Information
- Published on Friday, 05 February 2016 17:08
- Written by xiaobin
- Hits: 253
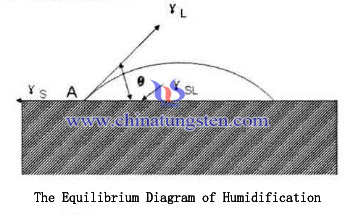
From the thermo dynamics of view, Equilibrated at the point of contact of the solid and liquid phases must meet γS = γSL + γLcosθ. θ is the wetting angle or contact angle, when completely wet, θ = 0 °, then γS = γSL + γL; partial wetting, 0 °< θ < 90 °; completely non-wetting, θ> 90 °, this time γS ≥ γSL + γL. Liquid phase sintering occurs must satisfy θ < 90 °, only with complete wetting liquid or partially wet to penetrate the pores and grain gap, forming a network-like structure of the coating. If θ > 90 °, the liquid produced during sintering will quickly bleed sintered body, densification can not be successfully completed, so that the group of tungsten copper alloy phase changes occurs.
View from the classical liquid-phase humidification theory, Only when we can see the surface energy of the solid and liquid phases is greater than the sum of the solid - liquid interfacial energy, that WSL = γS + γL - γSL in WSL> 0, when the liquid in order to effectively wet the solid surface. Solid phase has certain solubility in the liquid phase will help to improve wettability, promoting an increase in the number of liquid can be carried out by means of liquid mass transfer, and dissolved in a liquid phase in the process of dissolving precipitated solute portion may be carried out to fill the solid phase defects and particle clearance particle surface, thereby further improving the solid phase particle distribution uniformity. The quantity of liquid phase required to meet the solid particles fill the gaps, reduce the porosity of the material, increasing the density of the material, generally 20% -50% of the volume of liquid phase sintering of the best accounts, exceed prone to sintering deformation is insufficient so that the liquid can not be filled solid tungsten skeleton pore and solid particles come into contact with each other and the grains will grow up.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Difference Between Tungsten Disulfide and Molybdenum Disulfide
- Details
- Category: Tungsten Information
- Published on Monday, 15 February 2016 16:46
- Written by Cristina
- Hits: 263
Tungsten disulfide can take place of molybdenum disulfide in almost every field with even more wider application. Molybdenum and tungsten is in the same phrator of chemical element, chemical property of tungsten is more stable and higher molecular weight. Molybdenum is widely used due to its low price and high efficiency of marketing supplement. In fact, tungsten disulfide is not newly developed product, its application history is all the way with molybdenum disulfide. Due to its high price, it is firstly used in NASA aerospace, military, automobile industry and so on. A few years ago, price of tungsten disulfide is ten times higher than molybdenum disulfide. Nowadays price of molybdenum disulfide is increasing rapidly. And the price of these two materials is the same. So it would be more valuable if tungsten disulfide is used.
Differences between tungsten disulfide and molybdenum disulfide:
Note:PSI=6.89×103Pa
|
Property |
Tungsten Disulfide(WS2) CAS No 12138-09-9 |
Molybdenum Disulfide(MoS2) CAS No 1317-33-5 |
|
Color |
Silver grey |
Blue silver grey |
|
Appearance |
Crystalline |
Crystalline |
|
Melting Point(℃) |
1250~1260 (Decompose) |
1185 (Decompose) |
|
Boiling Point(℃) |
/ |
450 |
|
Density(Kg.m-3) |
7500 |
5060 |
|
Molecular Weight |
248 |
160.08 |
|
Friction Coefficient |
Static state0.07; Dynamic state0.03 |
/ |
|
Thermostability |
594oC(1100oF),COF <0.1 |
316oC(600oF),COF <0.1 594oC(1100oF),= 0.5 |
|
Carrying Capacity |
Coating 2000Mpa (300,000 psi);Under138Mpa(20,000 PSI),COF=0.044; Under138Mpa 2756Mpa (200,000~400,000 PSI ),COF reduce to 0.024 |
Coating 250,000 PSI |
|
Lubricant Temperature Range(℃) |
Environmental temperature: -273~650;Vacuum(10-14Torr): -188~1316 |
Environmental temperature : -185~350;Vacuum (10-14Torr): -185~1100 |
|
Chemical Stability |
Inert matters, odorless |
Inert matters,odorless |
|
Magnetism |
Non-magnetism |
Non-magnetism |
|
Electrical Characteristics |
Semi-conductor property |
/ |
|
Rockwell Hardness(HRC) |
30 |
/ |
|
Coating Thickness(Microns) |
0.5 |
/ |
|
Corrosion Resistance |
Slow down the corrosion process but not completely resist |
/ |
|
Coating Material |
Iron, steel, aluminum, copper, plastic and other artificial solid material |
Iron, steel, aluminum, copper, plastic and other artificial solid material |
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Electrode Liquid-phase Sintering Theory (1/2)
- Details
- Category: Tungsten Information
- Published on Friday, 05 February 2016 17:07
- Written by xiaobin
- Hits: 254
Tungsten copper electrode has both advantages of tungsten (W) and copper (Cu), such as high melting point, high density and high hardness of tungsten, excellent thermal and electrical conductivity of copper. Due to the big difference between tungsten and copper (the melting point of tungsten is 3387℃, the melting point of copper only 1083℃), it makes tungsten copper sintering become a typical liquid-phase sintering.
During the liquid-phase sintering process, Solid solubility in the liquid phase, the solid and liquid phases of liquid interfacial energy and the ability to penetrate the solid phase along the grain boundaries greatly affected the speed of change in the sintering and microstructure. In addition, the parameters, such as the granularity of particle distribution, powder purity, sintering temperature, sintering time, sintering atmosphere and the compact density are all the significant influencing factors of tungsten copper electrode. Liquid-phase sintering must meet wettability, solubility, phase number three conditions, the wettability of the solid, liquid surface tension (specific surface energy) interfacial tension γS, γL and two-phase (interfacial energy) γ are decided.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com