What Is the Standard Molar Enthalpy Change for the Reaction of Tungsten Diiodide?
- Details
- Category: Tungsten Information
- Published on Sunday, 09 July 2023 13:04
- Written by Shuangfeng
- Hits: 806
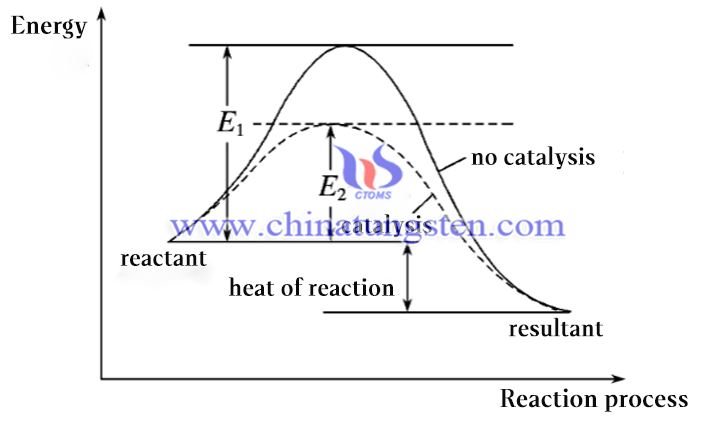
The standard molar enthalpy change for the reaction of tungsten diiodide (WI2) is -70.61 kJ/mol. it is defined as the thermodynamic energy change between the reactants (i.e., W and I2) required for the formation of 1 mol of WI2 and its corresponding reaction product (i.e., the WI2 gas) under the standard condition. Specifically, the enthalpy of formation of the reaction of tungsten diiodide can be determined by determining the standard molar enthalpy of formation of tungsten, iodine, and tungsten diiodide, a value that can be obtained by experimental measurement.
Read more: What Is the Standard Molar Enthalpy Change for the Reaction of Tungsten Diiodide?
What Is the Standard Molar Enthalpy of the Formation of Tungsten Diiodide?
- Details
- Category: Tungsten Information
- Published on Sunday, 09 July 2023 12:46
- Written by Shuangfeng
- Hits: 722
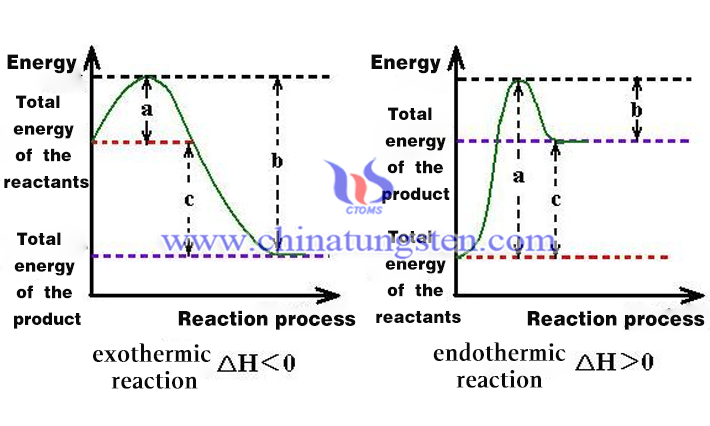
The standard molar enthalpy of formation of tungsten diiodide is -8.37 kJ/mol. The standard molar enthalpy of formation of tungsten diiodide, ΔfHmθ(WI2, g, 298 K), is defined as the standard molar enthalpy of formation for the reaction to produce the substance tungsten diiodide (νB=+1) from the monomers of the reference state at 298 K. The standard molar enthalpy of formation is -8.37 kJ/mol.
Read more: What Is the Standard Molar Enthalpy of the Formation of Tungsten Diiodide?
How to Obtain Tungsten Diiodide by Indirect Preparation?
- Details
- Category: Tungsten Information
- Published on Thursday, 29 June 2023 17:46
- Written by Shuangfeng
- Hits: 685
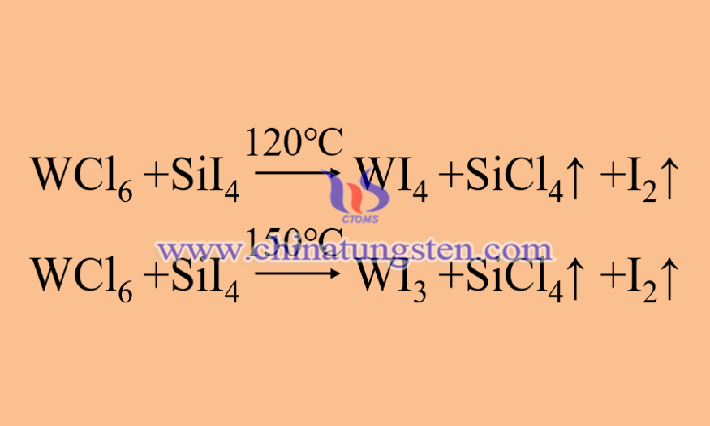
The indirect preparation method of tungsten diiodide does not aim at obtaining the product directly but is a method of preparing other tungsten iodide compounds before preparing the final product. The indirect preparation method develops based on the direct preparation method and chooses tungsten iodide compounds that are easier to prepare relative to tungsten diiodides, such as tungsten triiodide (WI3) and tetraiodide (WI4), as intermediate products, to reduce the requirements and uncontrollable factors of the reaction conditions and to improve the yield of tungsten diiodide.
Read more: How to Obtain Tungsten Diiodide by Indirect Preparation?
What Is the Type of Crystal Structure of Tungsten Diiodide?
- Details
- Category: Tungsten Information
- Published on Sunday, 09 July 2023 01:12
- Written by Shuangfeng
- Hits: 667
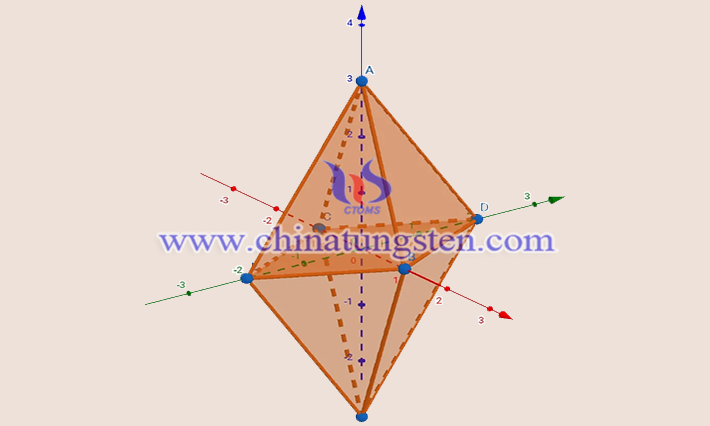
The type of crystal structure of tungsten diiodide is an orthorhombic crystal system, space group Bbem (No. 64), with lattice parameters a = 1258 pm, b = 1259 pm, and c = 1584 pm, and is a crystal cell with hexahedra. Each hexahedron has a tungsten atom in the center surrounded by six iodine atoms, forming a tungsten-iodine octahedron. The tungsten and iodine atoms are located at positions (0,0,0) and (0.5,0.5,0.5), respectively. The crystal has a layered structure with each layer consisting of tungsten-iodine octahedra, with neighboring layers interacting with each other through weak van der Waals forces.
Read more: What Is the Type of Crystal Structure of Tungsten Diiodide?
What Is the Tungsten Elemental Valence in Tungsten Diiodide?
- Details
- Category: Tungsten Information
- Published on Wednesday, 28 June 2023 17:15
- Written by Shuangfeng
- Hits: 780
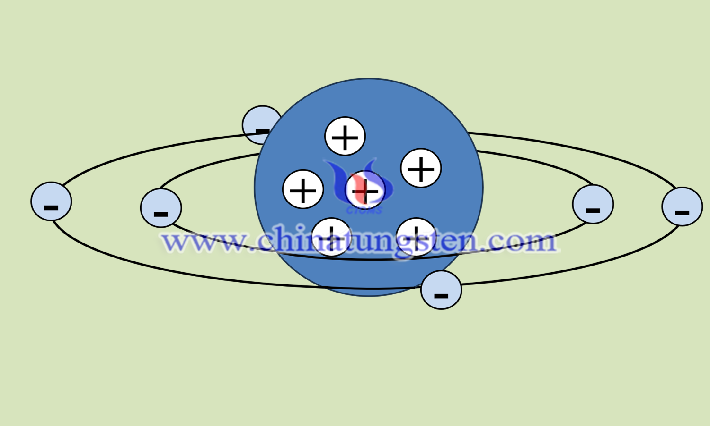
In tungsten diiodide, the tungsten element has a valence of +2, and the iodine element has a valence of -1. Therefore, the quantity ratio of iodine to tungsten in tungsten diiodide is 2:1, which is determined according to the principle of electroneutrality.
Read more: What Is the Tungsten Elemental Valence in Tungsten Diiodide?





 sales@chinatungsten.com
sales@chinatungsten.com