Evaluation of the Service Life of Tungsten Crucibles
- Details
- Published on Monday, 30 June 2025 11:23
- Hits: 155

Although tungsten itself has excellent thermal stability and corrosion resistance, the actual service life of tungsten crucibles is affected by a number of factors. Therefore, a lifetime assessment of the system not only helps to improve the efficiency of equipment operation, but also reduces material waste and maintenance costs.
Does the Machining Accuracy of Tungsten Crucible Affect Its Performance?
- Details
- Published on Monday, 30 June 2025 11:20
- Hits: 175

Tungsten crucible is one of the typical applications of tungsten material, in addition to the performance of the material itself, the influence of machining accuracy on the final performance of the crucible can not be ignored, especially in the context of increasingly strict requirements for high-precision manufacturing and thermal field control, processing quality has become one of the key factors to evaluate the reliability and practicability of the crucible.
Analysis of Heat Transfer Performance of Tungsten Crucible
- Details
- Published on Monday, 30 June 2025 11:17
- Hits: 168
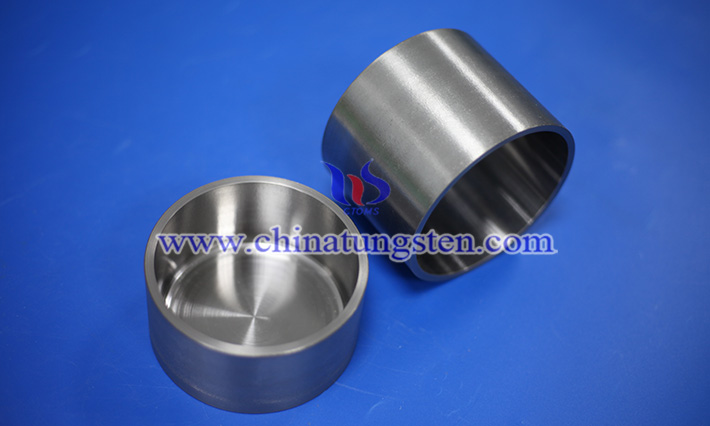
As an important part of the process of carrying molten metal, crystal growth, high-temperature evaporation, etc., tungsten crucible must not only have excellent high-temperature stability and chemical inertness, but also have good heat transfer performance to ensure the thermal efficiency of the process and the uniformity of temperature control.
Thermal Conductivity Analysis of Tungsten Crucibles
- Details
- Published on Monday, 30 June 2025 11:14
- Hits: 170

Tungsten crucibles are widely used in sapphire crystal growth, rare metal smelting, high-temperature material testing and other fields due to their excellent high-temperature stability and corrosion resistance.
Potential Applications of Tungsten Crucibles in the Nuclear Industry
- Details
- Published on Monday, 30 June 2025 11:11
- Hits: 160

Among many components, tungsten crucibles are attracting more and more attention from research institutions and industries due to their excellent heat resistance, corrosion resistance, radiation resistance and other characteristics, and its potential applications in nuclear fuel processing, high-level radioactive waste treatment and next-generation nuclear energy systems are worthy of in-depth discussion.
Chemical Stability Studies of Tungsten Crucibles
- Details
- Published on Monday, 30 June 2025 11:07
- Hits: 165

Tungsten material is widely used in high-temperature thermal field, vacuum smelting, crystal growth and other cutting-edge scientific and technological fields. In these applications, tungsten crucibles have become important vessels for carrying molten metals or chemical reactants due to their advantages of high temperature resistance, high thermal conductivity, and non-deformation.
The Powder Metallurgy Technology for Tungsten Crucible
- Details
- Published on Monday, 30 June 2025 11:04
- Hits: 159
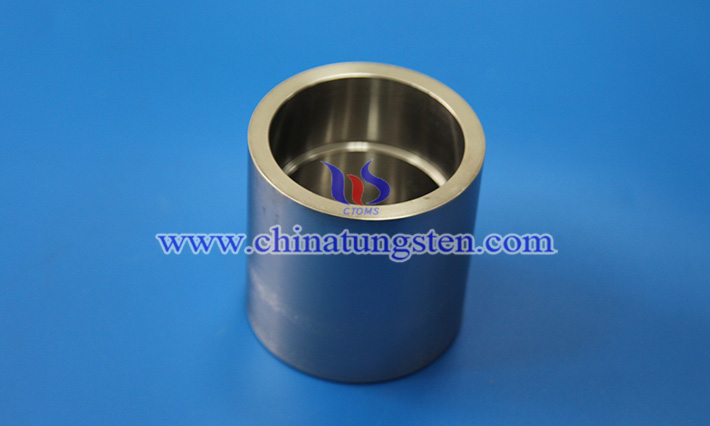
Tungsten is widely used in industrial scenarios such as high-temperature metallurgy, crystal growth, and vacuum smelting due to its ultra-high melting point, excellent high-temperature resistance and chemical stability. Especially in the preparation of molybdenum, titanium, rare earth and other active metal materials, tungsten crucible has become an ideal container material because it is not easy to react with the melt and can withstand extreme thermal stress.
Failure Analysis of Tungsten Crucible in High-Temperature Experiments
- Details
- Published on Monday, 30 June 2025 11:01
- Hits: 168

Due to its extremely high melting point, excellent high-temperature mechanical properties and good chemical stability, tungsten crucible is widely used in high-temperature experiments in aerospace, metallurgy, materials science and other fields, especially in crystal growth, metal smelting and rare element extraction, etc., which plays a key role. However, in the actual use process, tungsten crucibles are not "indestructible", and will still fail in different forms due to a variety of complex factors.
What Are the Surface Treatment Technologies of Tungsten Crucibles?
- Details
- Published on Monday, 30 June 2025 10:58
- Hits: 139
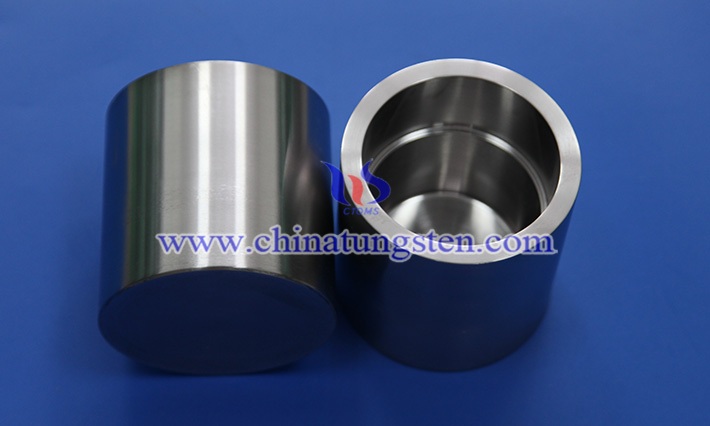
As a metal with a high melting point, tungsten is widely used in high-temperature smelting, single crystal growth, vacuum evaporation and other industrial fields due to its excellent thermal stability, corrosion resistance and high strength.
What Is the Wear Resistance of Tungsten Crucible?
- Details
- Published on Monday, 30 June 2025 10:51
- Hits: 156

Tungsten crucibles play an important role in high-temperature metallurgy, crystal growth, vacuum smelting and other fields, and have long been known for their excellent high temperature resistance and good chemical stability.



 sales@chinatungsten.com
sales@chinatungsten.com