Enhanced Activity of Urea Electrooxidation on Tungsten Carbide Composites Using APT
- Details
- Category: Tungsten Information
- Published on Friday, 09 July 2021 04:49
As a hydrogen carrier for long-term sustainable energy supply, urea (CO(NH2)2) has been attracting increasing attention due to its stable, relatively non-toxic, non-flammable and renewable properties. Recently, the electrooxidation of urea has been used as an effective approach for hydrogen production as well as treatment of urea-rich wastewater.However, the current major challenge is to improve the catalytic activity and CO-tolerance of the catalyst.
Tungsten carbide (WC) as the co-catalyst in the nickel-based catalyst can improve the catalytic activity and stability. In order to further improve the electro-conductivity as well as the loading of the active sites, multi-walled carbon nanotubes (MWCNT) are used as the carrier in this work. Therefore, Tungsten carbide composites has been prepared using APT with enhanced activity of urea electrooxidation.

The preparation process of the urea electrooxidation catalysts are as following steps:
First, MWCNT (2.0 g, >200 m2 g−1, made by the CVD method, from TIME NANO Co. Ltd.) was incipient impregnated in an ammonium paratungstate aqueous solution (containing 0.4 g (NH4)6W7O24·6H2O), followed by a temperature-programming reduction (TPR) treatment with CH4 and H2 to obtain WC/MWCNT framework (WC was 12.5 wt %). Then, these WC/MWCNT powders were added to a nickel nitrate aqueous solution (containing 2.8 g Ni(NO3)2·6H2O) with the impregnation and another TPR process to produce the final catalyst containing 20 wt% Ni, 10 wt% WC and 70 wt% MWCNT (noted as Ni-WC/MWCNT). All the TPR procedures were as previously reported [5]. For comparison, Ni nanoparticles were supported on the WC/C, MWCNT or the activated carbon by the same procedures at the same loading, noted as Ni-WC/C, Ni/MWCNT or Ni/C.
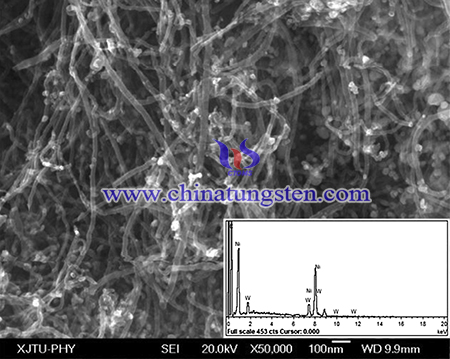
In conclusion, tungsten carbide composites has been prepared using APT with enhanced activity of urea electrooxidation. Furthermore, the electron-transfer resistance of the urea electrooxidation decreases from 12.6 Ω on Ni-WC/MWCNT to 8.5 Ω on Ni-WC/C due to the high electro-conductivity of MWCNT. the maximum current density at the Ni-WC/MWCNT electrode is 46.6 mA cm−2, which is over 3 times and 15 times higher than those of Ni-WC/C (12.3 mA cm−2) and Ni/C (3.1 mA cm−2).
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com