Preparation of Ammonium Paratungstate from Tungsten Alloy Scrap
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2021 21:58
Tungsten is one of the most highly refractory metals and is well applied in lamp filaments, heating elements in high temperature furnaces, electronic heaters, X-ray emission tubes, TIG welding electrodes etc. The widely applications of tungsten result from its thermal resistance to high temperature, high hardness, and high wear resistance.
As a valuable and expensive metal element. It is necessary to recover it from tungsten alloy scraps. Thus, a preparation process of ammonium paratungstate (APT) from tungsten alloy has been conducted.
The process to recover tungsten from tungsten alloy scraps is as below:
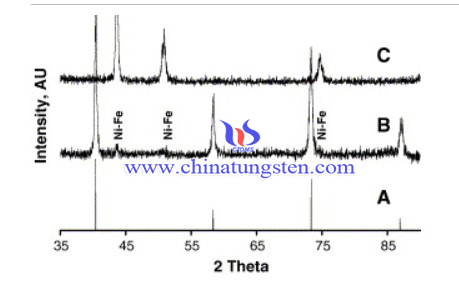
Firstly, APT, Mo, Fe and Ni powders were mixed and heated at 1300 °C in the presence of hydrogen and finally annealed in vacuum at 1000 °C. The tungsten alloy sample was obtained. The scrap was cleaned to remove all dirt, oil etc. and then used. The composition of the fresh scrap was W–88%, Ni–7.5%, Fe–4%, Mo–0.3%.
For anodic potential measurements the electrolytic cell set up consisted of a perforated PVC anode basket (dia 5 cm) packed with the scrap with a graphite rod as the current lead and a stainless steel (SS) sheet cathode of size 5 cm × 8 cm within a 500 mL beaker. The electrolyte employed was NaOH with and without additive, viz. chloride. The longer duration experiments were carried out in a similar set up, but larger in size. A cylindrical PVC tank (20 cm dia × 30 cm height) having provision for inlet and outlet served as electrolytic cell. The anode consisted of a perforated PVC cylinder packed with the scrap with a cylindrical graphite rod used as current lead, a SS sheet cathode was placed adjacent to the anode.
For continuous runs, fresh sodium hydroxide was fed from an overhead tank and the outlet containing sodium tungstate and sodium hydroxide was collected. The flow rate was adjusted so as to maintain 100 g/L Na2WO4 with 60 g/L NaOH in the electrolytic cell.

The tungsten alloy scrap with an exposed geometrical surface area of 0.3 Cm2 was used for the experiments in an H-type cell. Platinum gauze was employed as counter electrode. Potentials were measured vs. saturated calomel electrode (SCE) through a Luggin capillary and converted to NHE. The sodium tungstate solution was subjected to molybdenum removal step employing sodium hydrogen sulphide. The pH of the solution was adjusted suitably, tungstic acid was precipitated under optimum conditions and APT was prepared.
In summary, the result showed that the synthesized APT was of commercially acceptable quality. Anodic dissolution could advantageously be employed for dissolving tungsten from tungsten alloy scrap. The energy consumption for dissolution in NaOH electrolyte is approx. 2 kWh/kg. The addition of NaCl to NaOH electrolyte is not advantageous in the dissolution. Recycling of NaOH obtained through electrodialysis is possible.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com