Sulfur-Molybdenum Catalyst Turns Carbon Dioxide into Methanol
- Details
- Category: Tungsten's News
- Published on Tuesday, 05 July 2022 18:16
The Vienna University of Technology (TU Wien) has developed a new method for concerting carbon dioxide (CO2) into liquid methanol (CH3OH) with the help of a special sulfur-molybdenum catalyst material. This new technology has now been patented and expanded to industrial scale.
CO2 can be used most effectively where CO2 occurs in maximum concentration, such as in the exhaust streams of large industrial plants. The idea of converting CO2 into a valuable product is not new. However, this is a difficult and complex task. Sometimes, CO2 has to be enriched and separated beforehand, which requires additional costs and energy investments.
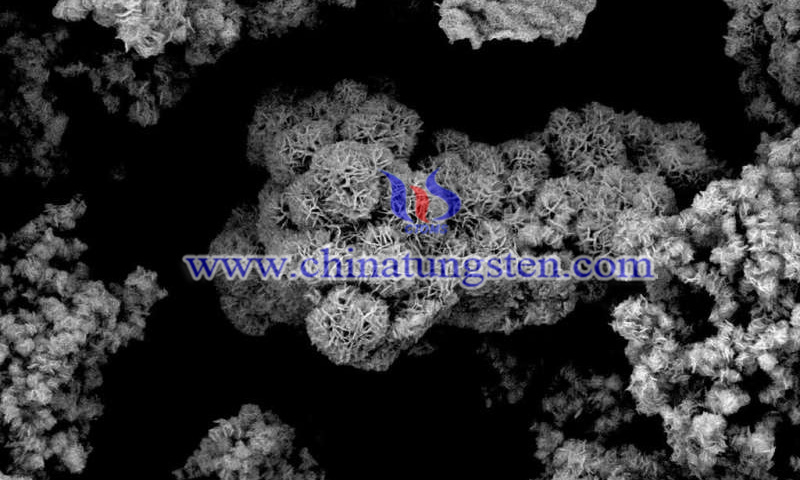
(Credit: Vienna University of Technology)
"To date, copper-based catalysts have often been used in order to convert CO2," says Prof. Karin Föttinger from the Institute of Materials Chemistry at TU Wien. "However, they have one major drawback, namely that they are not robust. If the exhaust gas stream has certain other substances in it besides CO2, such as sulfur, the catalyst quickly loses its activity."
Therefore, Karin Föttinger and her research team set out to find a better material. "If you want to use this method not only in the laboratory, but also on a large scale in industry, then you need a catalyst that may be a little less active, but that is robust, durable and reliable, and that does not require pretreatment for this engineering," Föttinger explains.
The research group at TU Wien has shown that sulfur-molybdenum catalysts can meet these requirements. Special additional elements, such as manganese, ensure that the inactive carbon dioxide is activated and converted. By selecting additional elements, the catalysts can be precisely adapted to the desired application area.
Methanol, also known as methyl alcohol, is a chemical and the simplest alcohol, with the formula CH3OH. It is a light, volatile, colorless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol. "CH3OH is liquid at room temperature and easy to store. Up to now, CH3OH has usually been produced from fossil feedstocks," says Karin Föttinger. "But it is also possible to use our catalysts to produce other molecules, such as higher alcohols. We are still trying to figure out how to choose the right parameters such as pressure and temperature to produce different products."
Karin Föttinger says: "This realistic method of converting carbon dioxide to methanol has been patented and will now be scaled up to industrial scale in cooperation with partner companies. In this way, this new sulfur-molybdenum catalyst should make an important contribution to achieving industry climate neutral."
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com