MoS2 Catalytic Hydrogenation of CO2 to Methanol
- Details
- Category: Tungsten's News
- Published on Friday, 02 April 2021 22:50
Scientists use sulfur vacancy-rich few-layered molybdenum disulfide (MoS2) to achieve low-temperature and high-efficiency carbon dioxide (CO2) catalytic hydrogenation to methanol. The greenhouse gas - CO2 is the final product of various chemical reactions. Excessive emissions have exacerbated the increase in global average temperature and brought tremendous pressure on the ecological environment. How to convert and utilize CO2 and turn it from waste into treasure is a research hotspot and difficulty in the field of energy and chemical industry.
Recently, the team of Dehui Deng, a researcher at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), and the team of Professor Ye Wang from Xiamen University have made important progress in the research of CO2 catalytic hydrogenation to methyl alcohol. The research team lasted nearly 6 years and for the first time used a low-layer molybdenum disulfide catalyst rich in sulfur vacancies to achieve low-temperature, high-efficiency, and long-life catalytic CO2 hydrogenation to methyl alcohol.
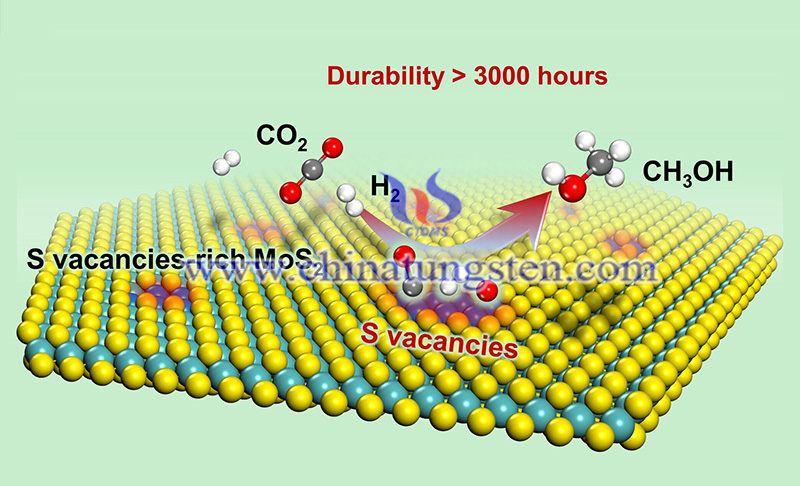
The MoS2 catalyst has significantly better activity and selectivity than previously reported metal oxide catalysts, and shows excellent stability, opening up a new way to achieve low energy consumption and high efficiency CO2 conversion and utilization. Related research results were published in Nature Catalysis.
Reacting with green hydrogen (H2) based on renewable energy to produce methanol is one of the important ways to turn CO2 waste into treasure. Generally, traditional metal oxide catalysts require a reaction temperature above 300 degrees celsius, and are often accompanied by severe reverse water gas shift reactions, thus producing a large amount of CO as the by-product.
The introduction of transition metal components in the metal oxide catalyst promotes the activation of H2 and reduce the reaction temperature, but it is easy to cause excessive hydrogenation of CO2 to produce methane, reducing the selectivity of the target product methyl alcohol. A new catalyst system is urgently needed for the low-temperature and high-efficiency hydrogenation of CO2 to produce methyl alcohol.
In previous research, Dehui Deng's team found that MoS2-based catalysts' excellent performance in catalyzing the electrolysis of water to produce H2. Conversely, "Can molybdenum disulfide activate H2 at room temperature and efficiently catalyze the hydrogenation of CO2 to produce methanol?"
With this idea, Deng' team will start joint research with Ye Wang's team. However, the initial research progress was not smooth. The catalytic performance of molybdenum disulfide did not achieve the expected results. The traditional methods of adjusting the catalyst performance such as introducing other elements into molybdenum disulfide also failed to significantly improve the catalyst performance.
The team first allowed molybdenum disulfide to react with H2 to generate a large number of sulfur atom vacancies on the surface, which in turn made the stable molybdenum disulfide surface lively. By modulating the structure of molybdenum disulfide itself, researchers have developed a few layers of MoS2 with abundant sulfur vacancies.
After evaluation, the molybdenum disulfide catalyst can realize the direct activation and dissociation of CO2 and H2 at low temperature or even room temperature, and effectively inhibit the excessive hydrogenation of methyl alcohol. The results of in-situ characterization and theoretical calculations show that the sulfur atom vacancies in the plane are the active centers for catalyzing the highly selective hydrogenation of CO2 to methanol.
The single-pass conversion rate of CO2 at 180 degrees Celsius can reach 12.5%, and the selectivity of methyl alcohol is as high as 94.3%, which is significantly better than the previously reported traditional catalysts such as metals and metal oxides. The performance can be stably maintained for 3000 hours without attenuation, showing excellent industrial application potential.
Dehui Deng said that the efficient conversion and utilization of CO2 is an important part of achieving 'emission peak' and 'carbon neutrality'. The research reveals the potential of the sulfur vacancies of two-dimensional molybdenum disulfide in catalytic reactions and provides new ideas for the development of new catalysts for CO2 hydrogenation. It is hoped that more companies can participate and jointly promote the industrial application of MoS2 catalytic hydrogenation to methanol.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com