What Is Tungsten Diiodide?—Ⅰ
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 June 2023 14:13
Tungsten diiodide(IUPAC name: Tungsten Diiodide, molecular formula:WI2) is an inorganic compound composed of tungsten and iodine after chemical reaction. The valence electron configuration of tungsten atom in tungsten diiodide is 5d46s2, which can form compounds with oxidation value from +2 to +6. Among them, the compounds with oxidation value of +6 are more stable. In tungsten diiodide, the valence state of tungsten is +2, and iodine is -1. So the molecule is composed of one tungsten atom and two iodine atoms.
The appearance of tungsten diiodide is usually black powder or block, with the density of 6.79 g/cm3 and the molar mass of 437.65 g/mol. Under normal temperature and pressure conditions, tungsten diiodide is insoluble in water, ethanol and carbon disulfide, but can be dissolved in alkali. Its structure is the same as tungsten dichloride, which is orthorhombic, space group Bbem ( No.64 ), lattice parameters a = 1258 pm, b = 1259 pm, c = 1584 pm.

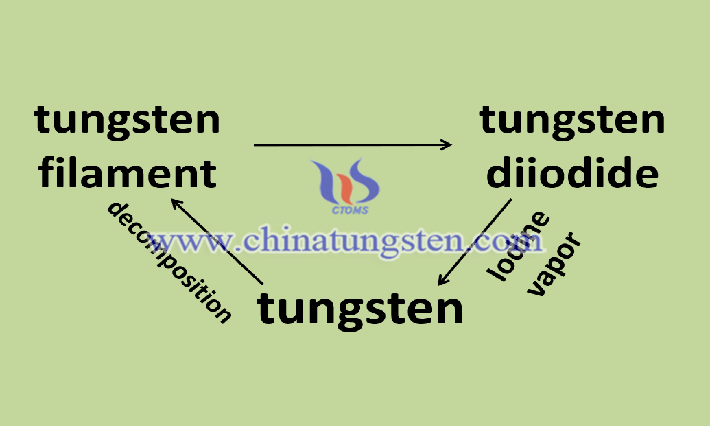
Based on the valence state of tungsten and iodine, tungsten diiodide can undergo oxidation and reduction reactions. First, it will be oxidized under heating to lose iodine and generate tungsten dioxide. Tungsten dioxide is an oxide of tungsten with a bronze-colored solid appearance. At the same time, tungsten diiodide can undergo hydrogenation reaction at 500 °C, and react with hydrogen to form metal tungsten. Under high temperature conditions, tungsten diiodide will directly decompose to form iodine and tungsten. This property can be applied in the field of lighting. Tungsten diiodide decomposes on the tungsten wire to leave tungsten, and iodine returns to the glass wall and reacts with the tungsten on the glass wall again to carry out the halogen tungsten cycle to prolong the service life of the iodine tungsten lamp. Tungsten diiodide can also undergo a substitution reaction, such as tungsten diiodide and bromine undergo a substitution reaction at 350 °C to produce tungsten dibromide. Tungsten dibromide is similar to tungsten diiodide and can be used as an alternative to some catalysts and electrode materials.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com