Tungstate Titanium Oxide Nanotubes Improve Ion Exchange in Fuel Cells
- Details
- Category: Tungsten Information
- Published on Wednesday, 26 October 2022 21:28
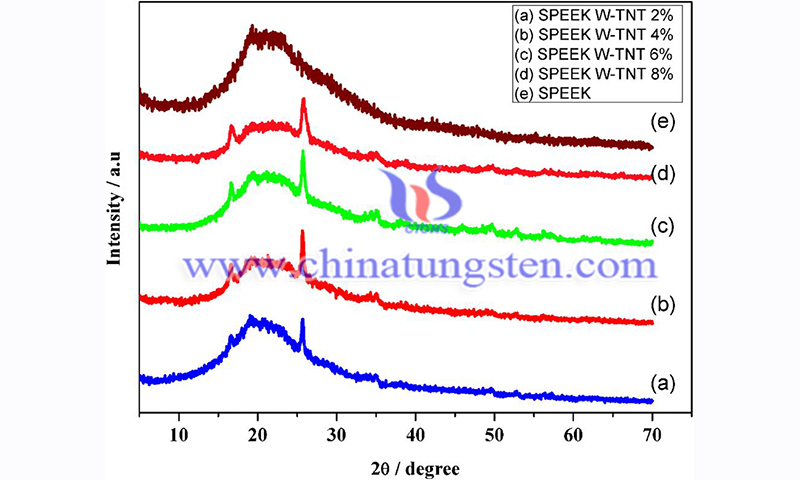
Ion exchange capacity is a vital property of ionic membranes, and this property is enhanced with the addition of ion-exchange materials. The IEC value of pure SPEEK membranes is 1.9 meq g-1 due to the contribution of sulfonate group (SO3H). increasing the content of tungstate titanium oxide nanotubes (W-TNT) in sulfonated poly ether ether ketone (SPEEK) membranes could improve the fuel cell ion-exchange capacity.
The increase in ion exchange is attributed to the presence of ion-exchange sites (tungstic acid) in TNT, which is involved in the ion-exchange process in addition to the sulfonic acid group of SPEEK. Even the addition of 2 wt% W-TNT increases the IEC value from 1.9 meq g-1 to 2.5 meq g-1, which implies that a large amount of tungstic acid is present in TNT.
The maximum IEC value of SPEEK 6% W-TNT composite membrane was 3.2 meq g-1. While SPEEK 8% W-TNT showed a decrease in IEC value, which may be due to the aggregation of fine particles that prevented the ion exchange group from participating in the ion-exchange process. Water molecules inside the membrane play an important role in proton conduction. The higher the water absorption of the membrane, the more it facilitates proton diffusion. In general, the water uptake of the membrane increases with IEC due to the increase in hydrophilicity.
The hydrophilic W-TNT filler enhances the water-holding capacity of SPEEK membranes. As a result, all composite membranes have higher water absorption values than pure SPEEK. The largest water absorption value is shown by the SPEEK 6% W-TNT composite membrane (19.24%), a value that is more affordable for membranes for fuel cells operating in wet conditions.
The purpose of incorporating W-TNT packing particles in SPEEK membranes is to enhance proton transport within the composite membrane, which can be achieved in two ways: The hollow nature of tungstate titanium oxide nanotubes can retain a large number of water molecules within the membrane (due to its tubular morphology), thus enhancing the water-mediated proton conduction pathway (Grotthuss mechanism).
In addition to the sulfonate group of SPEEK, the ion exchange group (tungstate) in W-TNT can also participate in the proton exchange process (hopping mechanism). This combined effect makes the composite membranes less resistant to proton movement. As a result, all composite membranes show higher electrical conductivity than the plain membranes.
The homogeneous mixing of the filler with the polymeric film resulted in an increase in the amorphous nature, which further contributed to the proton conductivity. The membranes with 6% filler concentration exhibited maximum conductivity compared to other membranes. A slight decrease in conductivity was observed for 8 wt% of the membranes containing filler due to the aggregation of nanotubes that hindered the ion channels in the polymer membranes.
The tensile strength of the composite membrane is higher than that of the SPEEK membrane due to the addition of W-TNT fillers. The increase in tensile strength is due to two factors, firstly, the membrane has been reinforced with these inorganic fillers, and secondly, the hydrophilic nature of the fillers increases the water uptake, which leads to an increase in elastic properties.
The best tensile strength of 22.1 MPa was obtained with 6% W-TNT in SPEEK, due to the most uniform distribution of W-TNT without any agglomerates, which helped to spread the applied stress throughout the membrane. When the percentage of W-TNT exceeded this optimum level, i.e., 8%, the strength of the membrane decreased to 19.6 MPa, due to the higher concentration of filler causing stresses to concentrate at the interface of the W-TNT/SPEEK composite.
Cited paper: Elumalai V, Deenadhayalan T, Kathleen Asitha A, et al. Preparation of tungstic acid functionalized titanium oxide nanotubes and its effect on proton exchange membrane fuel cell[J]. SN Applied Sciences, 2019, 1(4): 1-12.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com