Colloidal Tungstic Acid-III
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:26
Colloidal solutions of tungstic acid, in presence of various organic reducing agents such as formaldehyde, sucrose, glucose, dextrin, etc., yield intensely blue solutions on exposure to light. If the solution is kept for some time, it does not undergo this reduction on being exposed to light; but on raising the temperature the blue reduction products are obtained. In order to account for this it has been suggested that two forms of colloidal tungstic acid exist, one being photochemically sensitive and the other not. The former changes spontaneously into the latter, the reverse change being brought about by rise in temperature, and the absorption spectra of the two modifications differ considerably.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Colloidal Tungstic Acid-II
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:23
The colloidal solution may also be prepared by dissolving tungsten tetrachloride in alcohol and ether (equal volumes) and then diluting with alcohol and water. The solution obtained acts as a positive colloid and coagulates immediately when small quantities of neutral salts, hydroxides, or strong acids are added. On passing an electric current through the solution, a deep blue precipitate separates at the cathode.
A hydrosol of tungsten hydroxide is readily produced by the electrolysis of a 2 per cent, solution of sodium tungstate between a mercury cathode and a silver anode in a Hildebrand cell. The solution must not be allowed to become acid, or blue compounds are produced. The hydrosols obtained in this way are clear and transparent but brown in colour. The addition of potassium chloride causes coagulation, a black powder, resembling the lower oxides of tungsten, being formed.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-VII
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:11
Such formulation suggests a closer relation to the metatungstates than appears to be justified, and would not account for the very essential differences between the two types of compounds.
Paratungstates gradually decompose in aqueous solution with formation of the normal and metatungstates, so that while a freshly prepared solution is neutral to phenolphthalein, it gradually becomes acid on standing - more rapidly on boiling. For this reason the electrical conductivities of the solutions slowly increase at ordinary temperatures.
According to Hallopeau the free paratungstic acid is formed in dilute solution when the barium salt is treated with dilute sulphuric acid. Concentration of the solution, even in vacuo, causes decomposition, and on prolonged boiling, metatungstic acid is formed. Alkalies neutralise the solution, yielding paratungstates. There is, however, no proof that this solution contains any special modification of tungstic acid.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-VI
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:07
The content of base to acid in paratungstates was first given by Laurent as 5R2O:12WO3, whilst Lotz and Scheibler suggested the formula 3R2O.7WO3.xH2O. Marignac, after careful analysis, concluded that most paratungstates contained 5R2O:12WO3, but that a few contained 3R2O:7WO3. Other investigators, for reasons mentioned above, were unable to decide between the two formulae. Copaux, from the behaviour of the salts towards dehydration, considered them to be hydrotungstates and gave them the co-ordinative formula R•5[H(W2O7)3].aq. In support of this he points to the fact that the paratungstates resemble the complex tungstates in absorbing ultraviolet light, whereas normal tungstates do not do so. Rosenheim suggests that they are 6-tungsto-aquates of composition R5H5[H2(WO4)6]aq.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-V
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:04
Solutions of tungstates containing ammonium sulphide yield with hydrochloric acid a brown precipitate of tungsten trisulphide. The addition of zinc chloride to a tungstate solution produces a yellow precipitate which becomes blue on warming with dilute hydrochloric or sulphuric acid. When excess of hydrochloric acid is added to a solution of alkali tungstate and the mixture reduced by means of zinc, brilliant colours, from red to blue, are produced; if phosphoric acid is used, a fine blue precipitate results.
The paratungstates are generally obtained by treating solutions of alkali normal tungstates with acid, or by double decomposition. They can only be obtained from solutions, and always contain water which appears essential to their constitution; it can only be removed with difficulty,.strong heating being necessary for complete dehydration, which is accompanied by decomposition of the salt into the soluble normal salt and the insoluble tetratungstate. From an investigation of the dehydration of the sodium and potassium salts the following results were obtained:
| Temperature, t° C. | Water remaining after heating at t° C. | |
| Na10W12O41.28H2O. | K10W12O41.11H2O. | |
| 110 | 5.0 molecules | 5.4 molecules |
| 150 | 4.0 molecules | 4.4 molecules |
| 200 | 2.4 molecules | 2.4 molecules |
| 250 | 1.4 molecules | 1.4 molecules |
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-IV
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 10:01
The normal tungstates of the alkali metals are usually obtained by fusing together tungstic anhydride and the alkali hydroxide or carbonate in equivalent proportions. Those of the heavier metals are produced either by double decomposition in solution, or by fusing together an alkali tungstate and the chloride of the metal, often in the presence of sodium chloride. The tungstates of the alkali metals and of magnesium are soluble in water, those of other metals being insoluble, or only slightly soluble, not only in water but also in dilute acids. Concentrated mineral acids (except phosphoric acid) decompose them, with separation of tungstic acid. In this reaction the paratungstates behave similarly, whereas the metatungstates are not decomposed.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-III
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 09:59
Of the numerous types of salts of ordinary tungstic acid only two (the normal, of composition R2O.WO3.xH2O, and the so-called para-tungstates in which the ratio R2O:WO3 = 3:7 or 5:12) have been accurately investigated and their existence established beyond doubt. The readiness with which one type of salt is transformed into another, and the fact that paratungstates decompose on prolonged contact with water or on heating, make exact analysis almost impossible; and an added difficulty lies in the high atomic weight of tungsten, the difference in composition of various compounds with high tungsten content being very small. It is from such causes that, although the paratungstates are recognised as a well-defined series of salts, their actual constitution and relation to the normal tungstates remains unestablished. Further, the higher acid salts such as hexa- and octa-tungstates appear to show a closer relation to meta-tungstates than to ordinary tungstates, but the nature of this has not been determined. According to Smith, tungstates of the type 4R2O.10WO3.xH2O constitute another very definite series of salts.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Salts of Tungstic Acid-II
- Details
- Category: Tungsten Information
- Published on Thursday, 25 June 2015 09:56
This division into only two groups is justified by the fact that the metatungstates show marked differences both in properties and in ionic reactivity from those of the ordinary normal and acid tungstates, whilst the latter are very similar in their reactions. The transformation of normal tungstates into ordinary acid tungstates takes place readily, whereas the formation of metatungstates - by the addition of tungstic acid or other acids to tungstates - takes place only slowly and incompletely at ordinary temperatures. Further differences between the two types of salts are found in the peculiar behaviour of metatungstates on dehydration, and in the fact that whilst normal and para-tungstates increase the specific rotatory power of tartaric acid, the metatungstates do not act in this way.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Anti-radiation Shielding Made Of Tungsten Resin
- Details
- Category: Tungsten Information
- Published on Wednesday, 24 June 2015 18:12
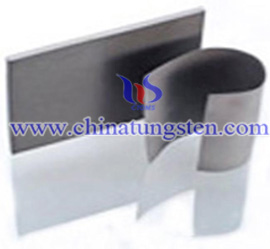
Tungsten resin, also known as tungsten poly, tungsten rubber, tungsten plastic, etc., which is made by the composition of various resins and tungsten powder mixed together through special metallurgical technology.
This type of tungsten resins may include ABS (acryloynitrile butadiene styrene), PP (polypropylene), PBT (polybutylene terephthalate), PA (polyamide), PU (polyurethane), and TPE (thermoplastic elastomer), etc.. As its special high density of 11.34 g/cm3 min., tungsten resin has a perfect radiation shielding performance comparable to lead for anti-radiation shielding, and it is much healthier. Besides, tungsten resin is very easy to be cut or holed with household scissors and so much formable into shapes with various curved surfaces.
For the above properties, and also it enables to be formed in 3-dimensional mould, tungsten resin is popular for medical industry radiation protection, which is trying to replace lead radiation shielding products now, such as the fields of x-ray inspection, radiation-protective equipment, vibration suppression, acoustic (audio) isolation, etc.
Tungsten Alloy Manufacturer & Supplier: Chinatungsten Online- http://www.tungsten-alloy.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn
Sodium Tungstate
- Details
- Category: Tungsten Information
- Published on Wednesday, 24 June 2015 17:31
Sodium tungstate is the inorganic compound with the formula Na2WO4. This white, water-soluble solid is the sodium salt of tungstic acid. It is useful as a source of tungsten for chemical synthesis. It is an intermediate in the conversion of tungsten ores to the metal.
Tungsten powder Manufacturer & Supplier: Chinatungsten Online - www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn


 sales@chinatungsten.com
sales@chinatungsten.com