Tungsten Bronze Niobate Crystal I
- Details
- Category: Tungsten Information
- Published on Thursday, 21 April 2016 18:10
Many niobate crystals with tungsten bronze structure (TB) are excellent photorefractive materials, such as strontium barium niobate (SrxBa1-xNb2O6, SBN), lead barium niobate (Pb1-xBaxNb2O6, PBN) , lithium potassium niobate (K2Li2-xNb5 + xO15 + 2x, KLN), barium sodium niobate (Ba2NaNb5O15, BNN), strontium barium potassium sodium niobate ((K2Na1-x) 2 (SryBa1-y) n-2Nb2O6 KNSBN) newly-grown calcium and strontium barium niobate (CaxSryBa1-x-yNb2O6, CSBN), etc. Since there are a lot of space in its internal structure, the quality of these crystal materials can be further improved by molecular design or doping or to change their each kind of properties (such as photorefractive characteristics of crystal, etc.). In addition to water-insoluble and stable physical and chemical properties, the majority of tungsten bronze niobate also has excellent electro-optical or nonlinear optical properties. Such as a relatively large electro-optic coefficient and relatively low half-wave voltage. In view of these excellent properties of niobate with tungsten bronze structure, it is being widely used in laser frequency, electro-optic modulator, associative memory storage, optical information processing, superconducting, humidity sensors, solid fuel cells and other fields.
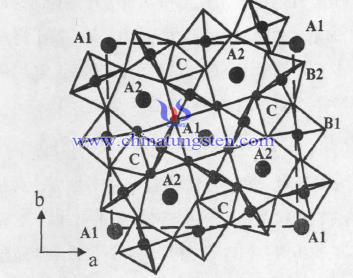
General chemical formula of the tungsten bronze structure crystal is AxB10O30, A can be cation with valent of one, two, three; B can be Nb, Ta, Ti, W and other positive ions, depending on the x, it may be classified as monoclinic, orthogonal, tetragonal crystals, hexagonal and cubic structure. The tungsten bronze structure generally called mainly refers to the tetragonal tungsten bronze structure. Structural formula of tungsten bronze niobate crystals can also be written as (A1) 2 (A2) 4 (C) 4Nb10O30: Nb - O octahedron constitutes lattice framework, and there are three different gaps of respectively with position 12 of A1, position15 of A2, position 9 of C. Gaps of A1, A2 and C can be filled with different valence of cation, to form a variety of compounds of tungsten bronze structure.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com