Synthesis and Characterization of Molybdenum Carbide Catalysts on Different Carbon Supports
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2022 22:51
The synthesis and characterization of molybdenum carbide catalysts on different carbon supports were investigated by researchers from Henan Polytechnic University. Molybdenum carbide was prepared by carburization of ammonium molybdate on cellulose, g-C3N4, MWCNTs, and sodium lignosulfonate without using any gaseous carbon source. In addition, the formation of molybdenum carbide was investigated in depth by in situ characterization using a simultaneous thermal analyzer-quadrupole mass spectrometry system.

Molybdenum carbide is a precious metal-like material that has shown great potential for many applications. Conventional Temperature-Programmed-Reduction (TPRe), which uses a carbon-containing gas with a molybdenum precursor for carburization, has made significant progress but is still difficult to scale up.
So far, transition metal carbides have become a hot topic in the field of new catalytic materials because of their unique electronic structure, high melting point, extreme hardness, and excellent catalytic properties. In general, transition metal carbides are interstitial compounds, formed by the insertion of carbon atoms into the lattice of transition metals, and exhibit the same special structure and physicochemical properties as expensive precious metals such as platinum.
Among the different transition metal carbides, molybdenum carbide has some unique properties and shows excellent catalytic performance in reactions such as selective isomerization of hydrocarbons, hydrodeoxygenation of phenols (HDO), ammonia synthesis, hydrodenitrogenation, water-gas conversion, hydrodesulfurization, hydrorefining, synthesis gas to lower alcohols and hydrogen evolution reaction (HER).
Typical synthetic processes are achieved primarily through the pyrolysis reaction of carbon and molybdenum precursors. Most methods are used to prepare molybdenum carbide catalysts temperature-programmed reduction (TPRe), carbothermic hydrogen reduction (CHRe), and carbothermal reduction (CRe), a single-source precursor method. In addition, chemical vapor deposition (CVD) and pyrolytic metal complexes have also been used to obtain molybdenum carbide at the nanoscale, high ratios, and clean surfaces.
Among them, TPRee uses molybdenum oxide as the precursor, carbon-containing gas (CH4, C4H10, CO, etc.) as the carbon source, and H2 as the reducing gas. This method allows the preparation of highly active catalysts, which burn easily in the air without passivation and are difficult to apply on a large scale. The problem with this method is the gaseous reactants, which are flammable and explosive and can easily cause accidents.
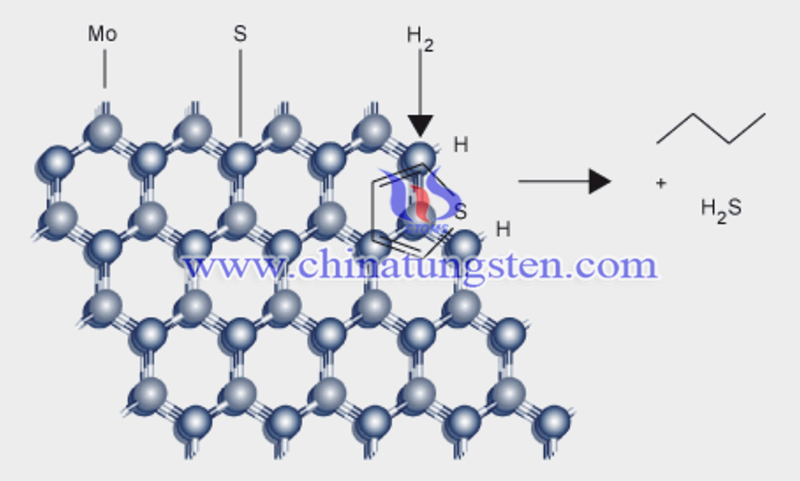
In fact, the presence of carbon supports not only provides a carbon source but also immobilizes and disperses the molybdenum source, thereby improving the dispersion of the prepared molybdenum carbide. Therefore, a solid carbon source is also a very critical factor in the production of molybdenum carbide in the carbon thermal reduction method.
In addition, various characterizations such as XRD, SEM, CO-TPD, FTIR and organic elemental analysis (OEA) have shown that molybdenum carbide prepared on different carriers can retain the skeleton and porous structure of the solid carbon carrier or generate new structures, and the composition and structure of the carrier can also have an impact on the adsorption and physicochemical properties of molybdenum carbide.
The study titled “Synthesis and Characterization of Molybdenum Carbide Catalysts on Different Carbon Supports” has been published in Catalysis Today (Pre-proof). The study was carried out by Shihang Menga, Xiaoxiao Xue, Yujing Weng, and Siyi Jianga et al. This study reveals the mechanism of formation of supported molybdenum carbide and provides a better understanding of the preparation of molybdenum carbide on different solid carbon materials.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com