WO3/BiOCl Heterostructure for Photocatalytic Degradation of Organic Pollutants
- Details
- Category: Tungsten Information
- Published on Tuesday, 05 October 2021 09:53
With the continuous development of industrialization, more and more attention has been paid to environmental pollution. Photocatalysis is an emerging and valuable strategy to purify water polluted by many environmental pollutants.
Bismuth oxychloride (BiOCl) is a p-type photocatalyst with a layered structure formed by [Bi2O2]2+ and chloride ions. Its internal self-built electrostatic field can significantly accelerate the distribution of photogenerated e--h+ pairs and improve the catalytic ability. Coupling with semiconductor oxides such as tungsten trioxide (WO3) is a common strategy to enhance photocatalytic performance.
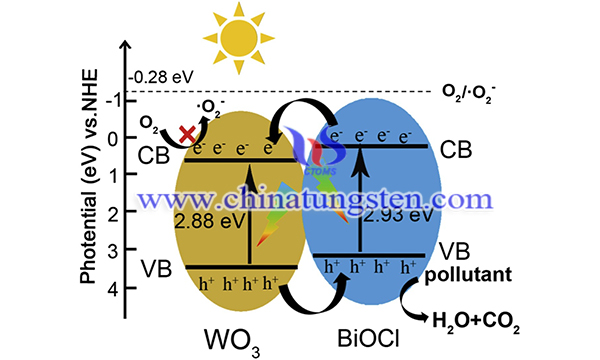
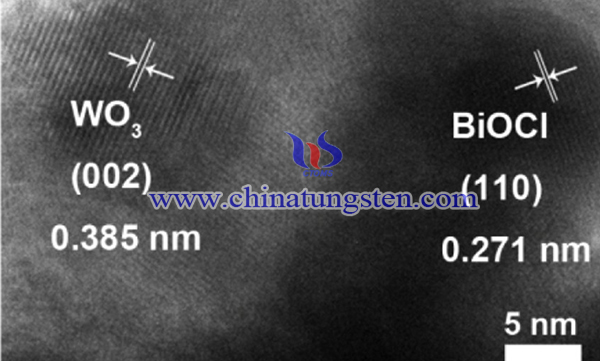
Thus, WO3/BiOCl heterostructure photocatalyst and the ultra-thin BiOCl nanosheets grown on WO3 nanofibers are synthesized by electrospinning and solvothermal methods. The WO3/BiOCl heterostructure shows an excellent performance in photocatalytic degradation of organic pollutants. The synthesis is as follows:
Polyvinylpyrrolidone (PVP K88-96, Mw=1,300,000), ammonium metatungstate hydrate (AMT, (NH4)6H2W12O40xH2O, 99.5%), N,N-dimethylformamide (DMF, AR), chlorine Bismuth (BiCl3, Trimethyl), ammonium bromide (CTAB, 99%), rhodamine B (RhB, AR), phenol (AR), CH3OH, ethylene glycol (EG) and acetonitrile (CH3CN) were used as raw materials without treatment.
WO3 nanofibers are synthesized by electrospinning and calcined in the air as follows. 1mmol (NH4)6H2W12O40·xH2O and 1.0g PVP were dissolved in 8mL DMF, stirred overnight at room temperature, and then transferred to a syringe placed in a syringe pump to obtain a precursor solution for electrospinning. The distance between the product collector and the needle tip of the syringe is set to 20 cm. The precursor nanofibers were obtained through the electrospinning process, when a positive voltage of 20 kV was applied to the needle tip and the solution feed rate was set to 0.5 mLh-1. Finally, the electrospun precursor nanofibers were calcined in a muffle furnace at a heating rate of 2°C/min at 500°C for 3 hours to obtain WO3 nanofibers.
Typically, 1 mmol WO3 nanofibers are dispersed in 20 mL ethylene glycol to form suspension A. At the same time, 0.2 g of CTAB was dissolved in 10 mL of ethylene glycol to form solution B. A certain amount of BiCl3 was dissolved in 10 mL of ethylene glycol to obtain solution C. Suspension A and Solution B were added to Solution C to obtain a homogeneous mixture, which was then transferred to an autoclave (50 mL) lined with polytetrafluoroethylene and kept at 120°C for 8 hours. After the reaction, the precipitate was collected by centrifugation, washed with distilled water and ethanol, and finally dried at 60°C to obtain WO3/BiOCl heterostructure. A series of WO3/BiOCl samples prepared by changing the BiCl3:WO3 molar ratio (0.5, 1, 2 and 3) are represented as WO3/BiOCl(0.5), WO3/BiOCl(1), WO3/BiOCl(2), WO3/BiOCl (3) Heterostructure.
In short, WO3/BiOCl heterostructure for photocatalytic degradation of organic pollutants has been successfully fabricated by growing BiOCl nanosheets on WO3 nanofibers through the electrospinning method followed by a solvothermal process. Compared with pure WO3 nanofibers and BiOCl nanosheets, WO3/BiOCl heterostructures exhibit strong light absorption and higher photogenerated carrier separation efficiency, so the decomposition of RhB and phenol under visible light is significantly enhanced Photocatalytic performance. The apparent rate constant of RhB degradation on the best WO3/BiOCl heterostructure is 0.259 min-1, which is about 2.3 times that of BiOCl nanosheets.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com