Graphene-WS2 Composite Prepared from Ammonium Metatungstate as Anode Material for Lithium-Ion Batteries
- Details
- Category: Tungsten Information
- Published on Thursday, 29 July 2021 19:41
A lot strategies had been applied to improve the enactment of lithium-ion batteries (LIBs) for next-generation electric vehicles (EVs). However, improving the energy density of LIBs is key extend the driving range of current EVs. Graphite has been broadly employed as the industrial standard LIBs anode material. However, graphite suffers from low theoretical capacity. Tungsten disulfide (WS2) is a transmission metal oxide which reveals trigonal prismatic structure and are indirect gap semiconductors in the bulk form with a bandgap of 1∼1.3 eV in the single-layer. This feature is used in a variety of fields, including energy storage, catalysis, solar cell, sensing, and electronic devices.

In order to overcome these drawbacks, graphene-WS2 composite is prepared using ammonium metatungstate (AMT), which is a promising anode material for lithium-ion batteries. The synthesis procedures of graphene-WS2 composite are as below:
0.8142 of AMT was dissolved in 15 mL deionized water and the solutions was then mixed with 100 mL graphene oxide (GO) solution (2 mg/mL). The mass ratio of GO to WS2 was corresponded to 1:4. The solution was then moved to polytetrafluoroethylene molds where they self-assembled to form a film at 80 °C in 24 h. The solution was reduced using hydrazine hydrate steam treatment to obtain the precursors. The precursor was calcined in a tube furnace (600 °C, 6 h) under nitrogen gas atmosphere to obtain WO3 and reduced GO (rGO) composites (G@WO3). Finally, the sample was mixed with thiourea with a mass ratio of 1:20 and then calcined at 850 °C for 3 h under nitrogen gas atmosphere. The graphene-WS2 composite was successfully synthesized.
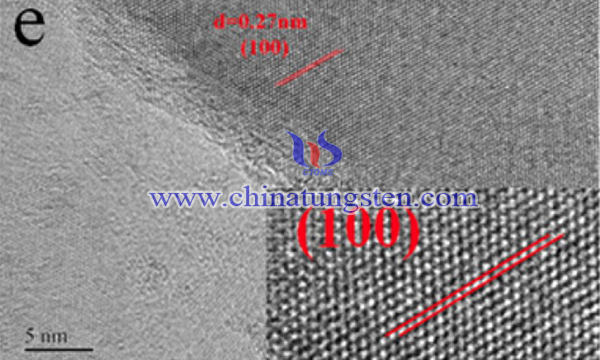
In summary, graphene-WS2 composite was successfully prepared from AMT as anode materials for lithium-ion batteries. The graphene-WS2 composite revealed high initial specific capacity (∼980 mAh g−1 at 0.1 A g−1), optimal cycling performance (∼450 mAh g−1 after 100 cycles at 0.1 A g−1), and superior rate capability (∼300 mAh g−1 at 2 A g−1). The graphene/WS2 mass ratio influenced the contact between WS2 and graphene and the porous nanofilm structure facilitated the kinetics of lithium-ion diffusion. The improved contact between graphene and WS2 has contributed to the remarkable properties of graphene-WS2 composite.
- AMT Manufacturer & Supplier, Chinatungsten Online: ammonium-metatungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com