WO3/Titania Nanotubes with Improving Photocatalytic Activity Fabricated Using Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Tuesday, 06 July 2021 21:45
Titanium dioxide (Titania) has been utilized in photocatalysis since the discovery of the early 1970s. Since then, researchers have developed a variety of methods to tune the nanostructure and the composition to optimize the photocatalytic efficiency. Compared with nanoparticles or the bulk materials, nanotubular-structure titania possesses larger specific surface area and stronger adsorption capacity that results to a better photocatalytic effect. These unique chemical and physical properties allow titania nanotubes (TNT) to be widely used in sewage treatment, air purification, and sterilization areas.
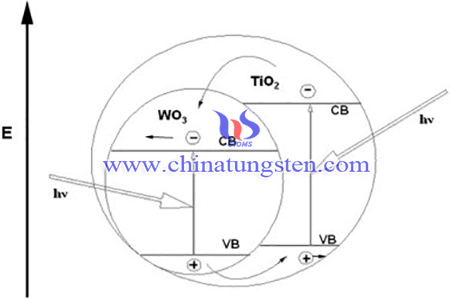
However, the facts that TNT has a wide energy gap along with a fast recombination rate of the photo-generated electron/hole pairs limit its applications. In order to improve the WO3/titania nanotubes with improving their photocatalytic activity, WO3/titania nanotubes had been fabricated using ammonium paratungstate. The results show that WO3 species are successfully coupled with TiO2 nanotubes, and the photocatalytic activity of WO3/TNT nanocomposites is strongly enhanced.
Degussa TiO2 (P-25) (average primary particle size is 21 nm and the specific surface area (BET) is 50 ± 15 m2/g), ammonium paratungstate (APT), NaOH, methyl orange (MO) were used as received.
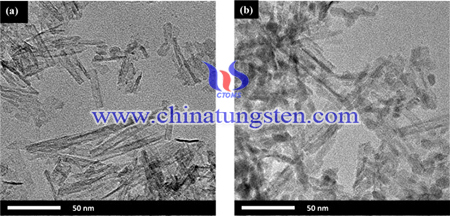
Firstly, 1 g TiO2 (P25) nanoparticles were added into 50 ml of 10 M NaOH aqueous solution, followed by hydrothermal treatment at 423 K for 24 h. After natural cooling to room temperature, the precipitates were centrifuged, collected, and washed with deionized water sufficiently until the pH value of the supernatant reached ca.7. Then the precipitates were immersed into 0.1 M HCl aqueous solution for 3 h and washed with deionized water until pH value of the rinsing solution reached ca.6. The final precipitates were dried at 383 K for 10 h and labeled by TNT(H). In order to obtain well-crystallized anatase TiO2, the dried TNT(H) were annealed in air at 673 K for 1.5 h at a rate of 3 K/min. These samples were labeled by TNT.
APT could be pyrolyzed into WO3 species at 383 K. The detailed fabricating procedures of the WO3/TNT composites are as follows: Desired amount of TNT precursors were mixed with APT aqueous solutions, which determined the atomic ratio of W/Ti = 0, 2, 6, 10, and 15%, respectively. The suspension was stirred magnetically for 2 h during the addition of stoichiometric 0.2 M HCl aqueous solution. The as-obtained precipitates were separated by centrifugation, and then washed with deionized water until the supernatant reached neutral, and finally dried at 383 K for 10 h. The samples with different W/Ti atomic ratio were labeled as W0T, W2T, W6T, W10T, and W15T.
WO3 nanoparticles were loaded on anatase TiO2 nanotubes through the reaction between (NH4)6W7O24·6H2O and aqueous solution of HCl. The obtained products were characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD) and UV–vis diffuse reflectance spectroscopy (UV-DRS). Degradation of methyl orange was employed to investigate the photocatalytic activity of the products.
In summary, WO3/TNT nanocomposites with enhanced photocatalytic activity were successfully synthesized. The optimal degradation rate of MO could reach 95% within 2 h irradiation. Results show that, among the samples with different concentrations of WO3, W10T with the atomic ratio W/Ti of 10% presents optimal degradation rate.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com