Synthesis of Nanosized Tungsten Carbide from Water Soluble Tungsten Source—APT
- Details
- Category: Tungsten Information
- Published on Monday, 05 July 2021 21:53
Thermal sprayed cemented carbides such as WC–Co(Cr) and Cr3C2–NiCr coatings are well known and widely used for wear protection purposes. Nanocarbides in cermets have shown promising results in gaining the hardness , wear performance in abrasion, cavitation or slurry type of wear conditions. Even friction properties have been reported to be improved by the use of nanocarbides. The driving force for reducing the carbide grain size comes from the fact that, as the carbide size becomes smaller, the binder mean free path is reduced, resulting in higher resistance to deformation and material loss. Many researchers have pursued such a hypothesis so as to improve the wear performance of HVOF WC–Co by reducing the WC grain size to the nanoscale.
Novel precursor synthesis methods include water-soluble raw materials and the reduction of tungsten oxide followed by carburization. Water-soluble tungsten source materials are typically ammonium paratungstate (APT) or ammonium metatungstate (AMT), and the carbon source is an organic compound or carbonising gas. Preparation of WC from water-soluble materials in a spray dryer and to follow the reaction sequences, starting from the raw materials throughout the WC synthesis steps from 20 to 1300 °C.
The preparation method of is as following steps: ammonium paratungstate ((NH4)10W12O41 5 H2O, APT) as tungsten source, and glycine (C2H5NO2) (molecular weight 75.07) as carbon source. Precursors were dissolved in distilled water by using a magnetic mixer with a heating unit. In the case of NS4, the first water was warmed up to 80 °C and then glycine was added and mixed until the solution was clear. Next, APT was added to the solution and mixed until dissolution was complete. APT was not fully soluble without glycine, but AMT dissolves fully. The precursors were dried with a laboratory scale spray dryer Buchi B-290. The spray dryer was used in order to ensure the homogeneity of the mixture and thus avoid separation of the raw materials during water evaporation. In spray-dried powders, the distance between the W-source and C-source can be at a maximum the diameter of the dried particle.
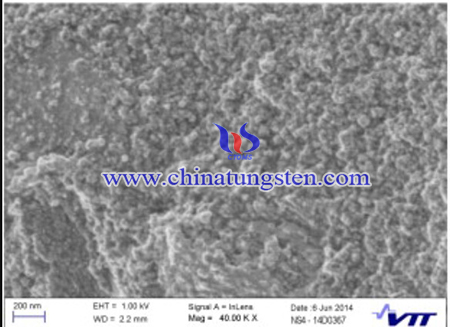
Heat treatments of the dried product were carried out under inert an Argon (Ar) gas atmosphere and under a reductive 4% Hydrogen in Argon (Ar-4%vol-% H2) gas atmosphere at 1300 °C so as to evaluate the effects of the heat-treatment atmosphere. Small-scale samples were heat-treated in a TG/DSC unit with a heating rate of 10 °C/min in the temperature range between 30 °C and 1300 °C without any holding time. Larger batches (5 g) for the carbon content measurements of precursors were handled in an inert gas tube furnace. In furnace trials, the heating rate was 3.3 °C/min to 400 °C, at which the holding time was 120 min. Because of the bigger batch sizes in the furnace compared to TGA (thermogravimetric analysis), the holding time was used to ensure sufficient time for released gas components to evaporate. Then the temperature was increased to 1300 °C with the same heating rate. The holding time at 1300 °C was 30 min.
In summary, Nanosized tungsten carbide have been synthesized from water soluble tungsten source—APT. 10–100 nanometre-sized WC have been with carbon source glycine in Ar- and Ar-4 vol-% H2-atmospheres at 1300 °C. From room temperature to 400 °C, APT lose ammonia and water groups and form presumably amorphous WO3, which later from 400 to about 700 °C reduce to metallic W.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com