Ammonium Paratungstate Applied in Libs and Investigation of the Durability
- Details
- Category: Tungsten Information
- Published on Thursday, 17 June 2021 04:19
The use of lithium ion secondary batteries (LIBs) as storage batteries for portable electronic devices and electric cars and as stationary storage batteries for solar cells and wind power generation is increasing yearly. However, their properties, such as energy density, power density, safety and durability, require further improvement to increase their use. Although graphite electrodes have been used as the negative electrode in LIBs, the durability is not long enough. Therefore, new materials for use as the negative electrode that can replace graphite have attracted interest. Transition-metal oxides are actively studied due to their good durability, high energy density and high-power density.
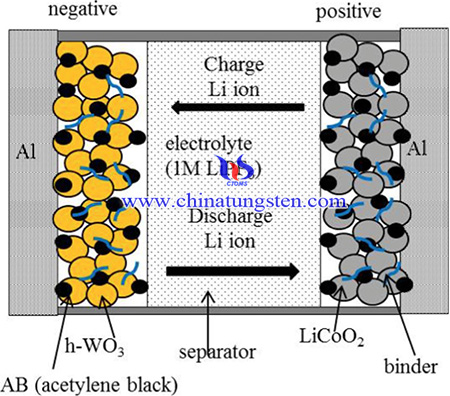
For these reasons, h-WO3 has the potential to achieve high energy density and power density in LIBs. LIBs fabricated with h-WO3 exhibit poor durability. Therefore, their improvement is strongly desired. The reason for the capacity decrease of LIBs using h-WO3 in charge-discharge cycling tests has not been well studied.
The synthesis method of the LIBs is as below: First, h-WO3 powder was prepared by the following process. Ammonium paratungstate (APT) solution was used as the tungsten source. Hydrochloric acid was added to the APT solution, the pH was adjusted to 7.3 and the mixture was left to stand at 2 °C for 48 h. Next, the mixture was stirred for 24 h at room temperature to induce the formation of the h-WO3 precursor. The obtained powder was filtered, then washed with deionized (DI) water, and dried at 120 °C for 2 h. Finally, the powder was annealed in air at 390 °C for 30 min to remove the hydrate W. The h-WO3 powder was mixed with acetylene black (AB) and a polyvinylidene fluoride (PVDF) binder in N-methylpyrrolidone (NMP) solvent in the weight ratio 100:5:5. The obtained paste was coated on an Al film (thickness: 15 μm) using a film applicator and annealed in air at 120 °C for 10 min to remove the NMP solvent. The thickness of the electrode was adjusted to 50 μm (weight per unit area: 10 mg/cm2). The LIBs in this study were composed of an h-WO3 negative electrode and a LiCoO2 positive electrode, which were separated by a 25 μm thickness polypropylene separator. The electrolyte was a 1 M solution of LiPF6 dissolved in a 50:50 vol% mixture of ethylene carbonate (EC) and diethylene carbonate (DEC). The assembly of the LIBs was carried out in Ar in a dry glove box.

In summry, the durability of h-WO3 when used as the negative electrode in LIBs. We carried out a charge-discharge cycling test in the voltage range 2.5–1.0 V (vs LiCoO2) for 500 cycles and clarified the degradation mechanism using in situ XRD, TEM, XPS, AAS and ICP-AES. The experimental results of the durability test were as follows. (1) Irreversible changes in the crystalline structure of h-WO3 occurred. (2) The deposition of a layer of reactive materials on the surface of the h-WO3 electrode and changes in the valence of W were observed. (3) Changes in the electrolyte composition occurred. The above results indicated the reasons for the degradation of h-WO3 rechargeable batteries.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com