Nanoscale Al/WO3 for Metastable Intermolecular Composites from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Saturday, 19 September 2020 14:58
Metastable intermolecular composite (MIC) materials is an intimate mixture of two or more compounds of which at least two are exothermically reactive. However, conventional thermites have a significantly lower reactive power. In contrast, MIC materials, while retaining the high energy density, can have a significantly higher reactive power than conventional thermites.
Investigators have conducted a synthesis method of nanoscale Al/WO3 for metastable intermolecular composites from ammonium paratungstate : creating ultrafine powders with particle sizes on the order of tens of nanometers. Reducing the particle size reduces heat and mass transfer length scales and increases the number of surface atoms per mass leading to significantly higher reaction rates.
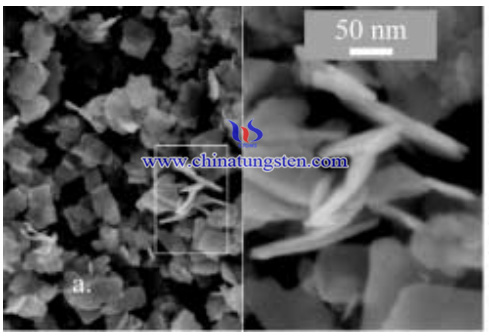
The specific process are as follows:
- Ammonium paratungstate, was dissolved in concentrated HCl in the ratio of 35 mg/ml by agitating ultrasonically. The solution was then rapidly poured into distilled water, causing the formation of a precipitate. The volume of water used was four times the volume of acid solution. The precipitate slurry was then subjected to several cycles of sonication, rinsing, and centrifuging. Finally, the solution was dried in air at 1008C forming a yellow powder. The powder was sieved to break up any macroscopic agglomerates. This procedure resulted in a hydrated WO3 (WO3 · H2O). Heating in air overnight at 200 ºC or 400 ºC removed water from the material and anhydrous WO3 was produced.
- aluminum as a fuel for reactive performance testing of tungsten oxide for this study provided nominally 44 nm Al powder. Preparation of MICs with a range of stiochiometries was performed by adding 326 ± 388 mg of WO3 to 10 cm3 isoproponal and de-agglomerating the suspension in an ultrasonic bath for 10 minutes. 112 ± 174 mg of Al was added to the WO3 suspension. This mixture was then agitated using a sonic probe (Sonifier 450) for 45 s at 50% power and 80% duty cycle. The solvent was removed from the resulting MIC formulation by drying on a hot plate coated with mold release at approximately 358C. A final sieving (200 mesh) step eliminated any macroscopic agglomeration. And the Al/WO3 MIC material was produced.
- Material was investigated using thermogravimetric analysis (TGA). The appropriate mass loss was calculated a priori from stoichiometry and the instrument was run under conditions optimized for each suboxide. When the mass loss reached the target, the process was quenched by inert gas and cooled. The resulting material was characterized using scanning electron microscopy (SEM), TGA, X-ray diffraction (XRD), Raman spectroscopy, and small angle X-ray scattering (SAXS).
In conclusion, a new high-performance MIC material called Al/WO3 was produced. The material releases 1 ± 2 MJ/kg of energy at a high rate of approx. 20 MW, which has been proved to be a nanoscale reactive MIC material.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com