Excellent Hydrogen Evolution Catalyst Using Ammonium Paratungstate as Raw Material
- Details
- Category: Tungsten Information
- Published on Saturday, 19 September 2020 15:15
The increasing use of fossil fuels is the major cause of global warming and climate change. Looking for a sustainable, clean, and highly efficient energy has become research hot spot.
In recent years, fuel cells and hydrogen are the two clean energies considered to be possible substitutes of fossil fuel. To produce hydrogen energy, splitting water by catalysis reaction is an ideal method. So far, the catalyst that has been developed includes most effective but expensive precious metals such as Pt and Pd Ill, oxides, sulfides, nitrates, phosphides, transition metal dichalcogenides, and various heterostructures. To overcome these disadvantages, an excellent hydrogen evolution catalyst named RGO/1T'-WTe2 using ammonium paratungstate as raw material has been conducted.
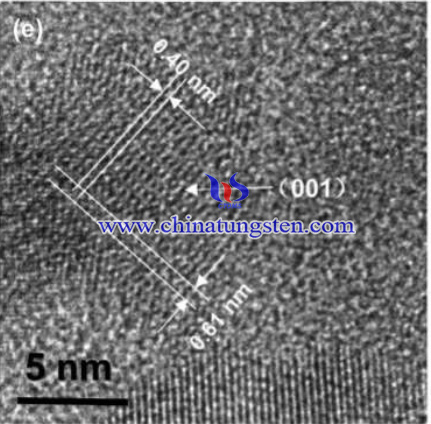
The detailed procedures are as below:
20 ml ammonium paratungstate (APT) aqueous so![]() lution with a W4+ concentration of 0.10 mol Il was made in deionized water. Similarly, a 20 ml W4+ precursor solution (0.10 mol./l) containing Cu2+ cations at the molar ratio of CulW = 0.01 was made by adding CuC12 9H20. Citric acid (CA, C6H807) (2.5 times molar of metal cations) as a cation chelating agent was added. At the same time, ultrafine tellurium powder, equimolar potassium hydroxide (KOH), and duple molars of potassium borohydride (KH4B) were reacted in deionized water in a sealed vessel for 96 h to form 5 ml Te2— aqueous solutions (0.8 mol.ll) at room temperature oc). Second, 20 ml cation solutions and 5 ml Te2— aqueous solution were transferred into a 30 ml hydrothermal reactor. After sealing, the hydrothermal reaction of the RGO/Wrre2 nanostrutures was successively carried out at 25 o c for 12 h, 50 oc for 3 h, 100 o c for 3 h, and then 160 o c for 14 h. After natural cooling to room temperature, dark brown powders were obtained by filtrate followed by washing repeatedly with deionized water. The powders were finally dried out in an electric furnace of 60 oc for 12 h. To synthesize the hybrids, 20 ml solutions W4+ precursor solutions were prepared in the mixed solution of deionized water and graphene oxide (GO) aqueous solution according to a mass ratio (0.02) of GO/V.rre-ž. For comparison, WTe2 was also synthesized with the same method in the absence of CA, RGO, and successive heating process.
lution with a W4+ concentration of 0.10 mol Il was made in deionized water. Similarly, a 20 ml W4+ precursor solution (0.10 mol./l) containing Cu2+ cations at the molar ratio of CulW = 0.01 was made by adding CuC12 9H20. Citric acid (CA, C6H807) (2.5 times molar of metal cations) as a cation chelating agent was added. At the same time, ultrafine tellurium powder, equimolar potassium hydroxide (KOH), and duple molars of potassium borohydride (KH4B) were reacted in deionized water in a sealed vessel for 96 h to form 5 ml Te2— aqueous solutions (0.8 mol.ll) at room temperature oc). Second, 20 ml cation solutions and 5 ml Te2— aqueous solution were transferred into a 30 ml hydrothermal reactor. After sealing, the hydrothermal reaction of the RGO/Wrre2 nanostrutures was successively carried out at 25 o c for 12 h, 50 oc for 3 h, 100 o c for 3 h, and then 160 o c for 14 h. After natural cooling to room temperature, dark brown powders were obtained by filtrate followed by washing repeatedly with deionized water. The powders were finally dried out in an electric furnace of 60 oc for 12 h. To synthesize the hybrids, 20 ml solutions W4+ precursor solutions were prepared in the mixed solution of deionized water and graphene oxide (GO) aqueous solution according to a mass ratio (0.02) of GO/V.rre-ž. For comparison, WTe2 was also synthesized with the same method in the absence of CA, RGO, and successive heating process.
Electrocatalytic hydrogen evolution performance was evaluated by electrochemical measurements on a YGCS electrochemical workstation (China). The measurement was performed in an H2S04 aqueous solution (0.5 M) that was Nrsaturated. The counter electrode was a PtlC wire, whereas the reference electrode was a-saturated calomel electrode. The working electrode was prepared by the following route: Graphite electrode (diameter: 5 mm)
In conclusion, the efficient charge transfer at the interface of the RGO and IT'-WTe2 led to the higher catalytic performances of hydrogen evolution reaction. Besides, doping Cu cation further resulted in the conduction enhancement and so enhanced activity and stability. This work suggested a simple approach to synthesize IT-WTe2 nanosheets and modify them with RGO incorporation and Cu doping to promote the advantages in their potential applications. An excellent hydrogen evolution catalyst named RGO/1T'-WTe2 using ammonium paratungstate as raw material has been proved to be successful.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com