Morphology of W18O49 Crystals by Microwave Decomposition of Ammonium Paratungstate (APT)
- Details
- Category: Tungsten Information
- Published on Thursday, 11 June 2020 00:03
Ammonium paratungstate (APT) as an important precursor in tungsten industry. It is the raw material for the majority of tungsten products. For example, tungsten trioxide (WO3), tungsten blue oxide, tungstic acid and ammonium metatungstate, etc. These are all intermediates by subsequent calcination and/or reduction of APT. Another significant middle product is monoclinic W18O49, (WO2.72), one of the tungsten suboxides, is an important intermediate product of WO3, influencing significantly the morphology of the tungsten powder end-product. W18O49 crystallises in needles and agglomerates of needle-like crystallites. This is true for the formation by vapour transport, in oxidative, and reductive atmospheres and for the transformation in the solid phase.
However, the main interest has been focused on its formation by gas phase reduction of higher oxides: WO 3 or WO3-x using H2, or CO, as reductive agents.
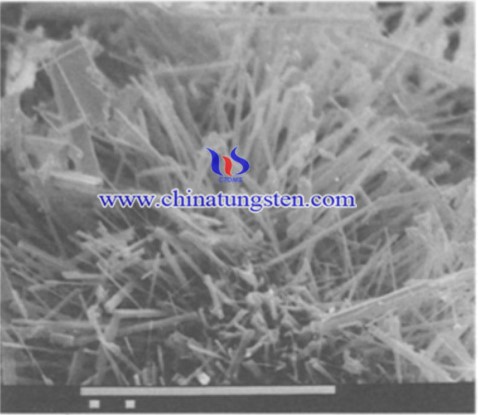
Microwave decomposition of APT has several significance for the morphological features and the growth mechanism of W18049. Since conditions during microwave decomposition of APT have been found favourable to the growth of W18049 whiskers. By understanding the morphology of W18O49 needle crystals, we can illuminate the function of whisker growth of tungsten oxide compounds. Scientists have conducted an experiment to clarify the morphology of W18O49 needle crystals prepared by microwave decomposition of APT.
The experiment procedures are as follows:
Samples were prepared by decomposition of APT under a neutral gas ambient in a 2.45 GHz microwave reactor. The microwave irradiation began with a slow increasing temperature of APT in the microwave reactor, later with a very quick increase temperature, then a rapid decomposition of APT occured. NH3 released from APT started to play the role of reductive agent and the reduction of WO3 came to an end after the escape of NH3. Released water of hydration causes large H20 pressure peaks in the gas phase confined in the pores of the tungsten oxide powder.
Quantitative X-ray powder diffraction was used in an attempt to By information on the amorphous fraction and on the crystalline phases. The morphology was studied by scanning electron microscopy as well as by transmission electron microscopy (TEM). For investigation by TEM the material was ground in an agate mortar, and a large number of small fragments were dispersed on carbon-coated specimen grids• Crystal structure of individual needles was established by electron diffraction. Attempts were made to reveal growth features and crystal defects by chemical etching of the samples using HF, ammonia water or K3FeCN 6 based etchants. Growth features were examined before and after chemical etching.
Conclusion
The results presented in this article demonstrate that the reductive formation of the needle crystals of W18049 occurs as a crystallisation process with the following main steps. (1) Root growth of a leader from a limited volume high mobility layer on the surface of the parent particle. (2) Growth in thickness with root growth of secondary needles parallel to the surface of the primary whisker combined with vapour deposition filling gaps. The model is suggested to explain morphologies of W18049 produced not only by microwave decom- position of APT but by reductions under wet conditions reported in the literature.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com