Catalytic Debinding in Injection Molding: Principle
- Details
- Category: Tungsten Information
- Published on Sunday, 22 October 2017 01:14
Degreasing principle of injection molding of catalytic debinding is to use a catalyst to organic carrier depolymerization of volatile small molecules, these molecules have a higher vapor pressure than other organic carrier molecules during debinding process, can quickly spread out the body. The binder system used in the process of injection molding cemented carbide catalytic debinding is generally composed of polyacetal resin, polymer with auxiliary framework and stabilizing additives.
Aldehyde Resin:
The structure of polyacetal resin is composed of repeated C-O bonds. It has strong adhesion, high gloss, high hardness, good weather resistance and chemical resistance. It is often used as tackifier resin to improve adhesion and hardness.
Reaction Process (as shown above).
The oxygen atom of the polymer chain is sensitive to the action of acid. When exposed to the appropriate acid catalyst, the catalytic reaction can be carried out at a lower temperature, resulting in the splitting of the macromolecular aldehyde resin into CH2O (formaldehyde) gas. Common catalyst gas used in catalytic degreasing process is nitric acid and oxalic acid gas.
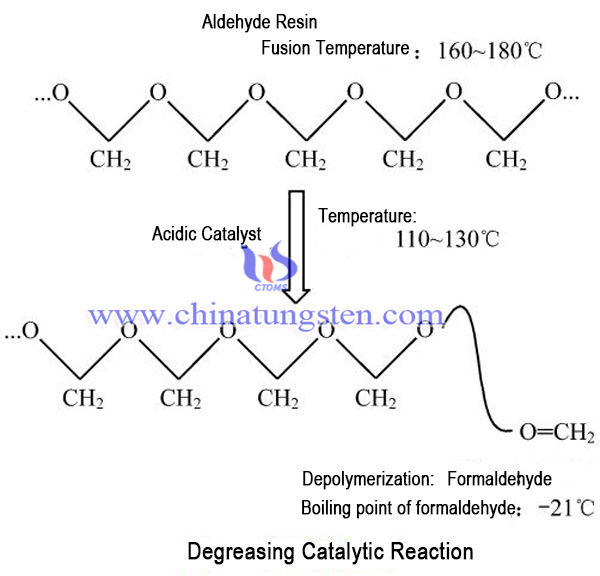
In the range of 110℃ ~ 130℃, the rate of Catalytic Debinding of cemented carbide can reach very high, and the temperature range is much lower than the melting interval (160℃~180℃) of the resin. Conditions for the catalytic reaction are particularly suitable for the removal of binder in powder injection molding. In this way, the main binder -- green poly formaldehyde resin in the presence of a catalyst directly from solid pyrolysis gaseous molecules, achieve rapid catalytic debinding.
At this point, a small amount of residual polymer (less than 10%) will form a shape preserving effect. The removal of the main green binder is still sufficiently strong, so that the parts have hard to continue in debonding, avoid any plastic deformation, better tolerance. Gaseous acid does not pass through the binder, and the reaction is only carried out on the interface between gas and binder. Gas diffusion in porous shell has been formed, the formation pressure in the interior will not green. These residues in green organic substances, decomposition agent, some polymers can be quickly in the pre-sintering stage by pyrolysis. Reaction equation is as follows:
〖(CH_2-O)〗_2 □(→┴(acid(T>100℃)) nCH_2=O)
- Tungsten Carbide Manufacturer & Supplier, Chinatungsten Online: tungsten-carbide.com.cn
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com