Stripping Method for WS2 Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 September 2022 00:45
- Written by Caodan
- Hits: 1616
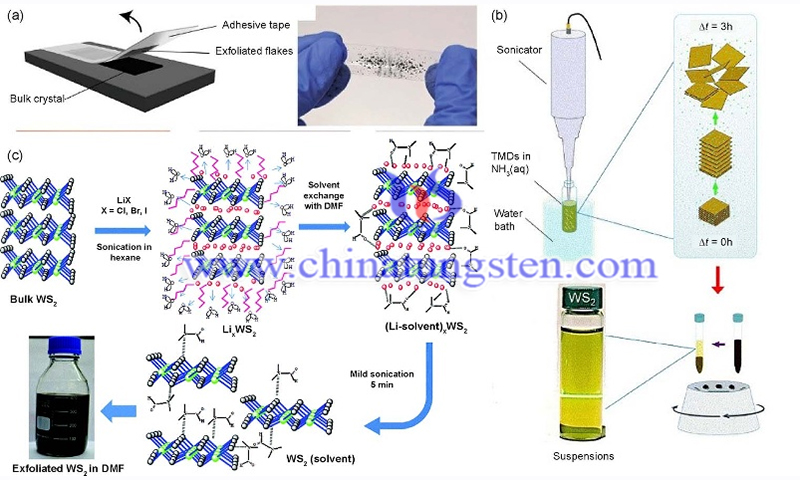
Bulk tungsten disulfide (WS2) can be stripped by physical and chemical methods, which are classified as mechanical and stripping method, and lithium-ion intercalation method. In recent years, in order to obtain large-area, high-quality monolayer tungsten disulfide films, researchers have tried to grow monolayer tungsten disulfide films on ingot substrates and then exfoliate them by atomic or molecular intercalation methods.
Chemical Methods for WS2 Film Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 September 2022 00:41
- Written by Caodan
- Hits: 1192
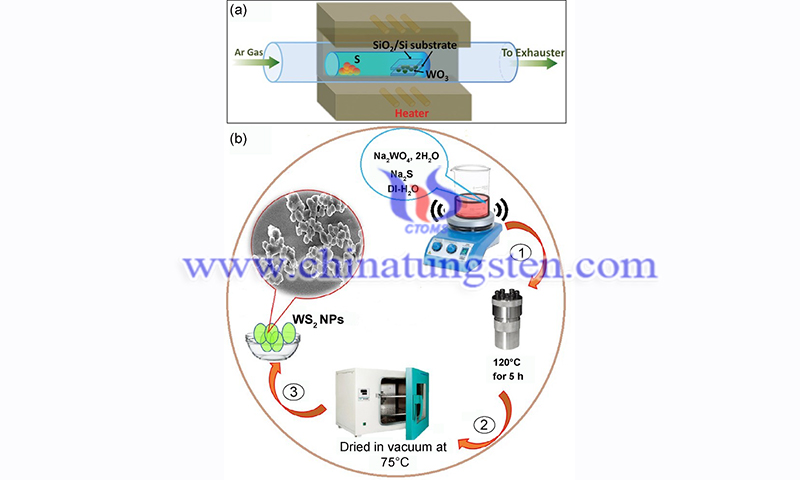
Two common methods for preparing tungsten disulfide (WS2) films by chemical methods are chemical vapor deposition (CVD) and hydrothermal growth of single-crystal tungsten disulfide from aqueous solutions under high temperature and pressure conditions. CVD is the most common method used to prepare tungsten disulfide. The CVD method involves a reaction process in which a gaseous precursor reacts chemically on a solid surface to produce a solid deposit.
Atomic Structure of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Sunday, 11 September 2022 22:22
- Written by Caodan
- Hits: 1544
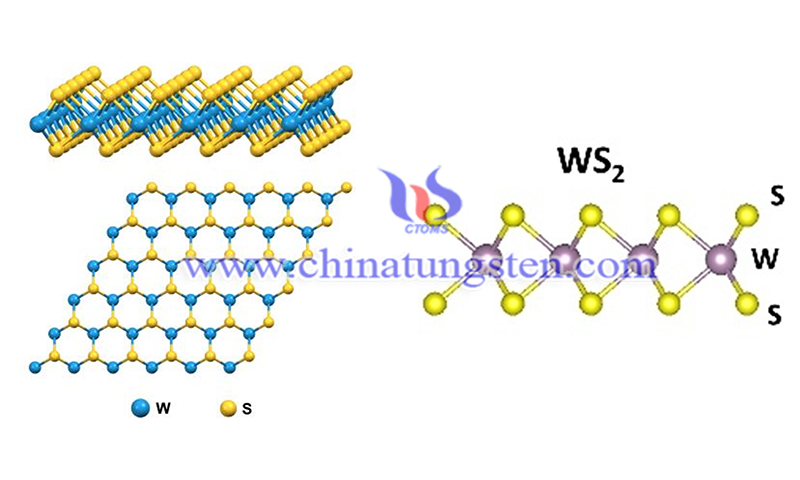
The atomic structure of tungsten disulfide (WS2) consists of a stack of three layers formed by a transition metal layer (W atom) sandwiched between two S-atom layers, each with a hexagonal lattice structure. In the three-layer stack, W and S atoms are bonded together by strong ion-covalent bonds. WS2 formed by these three layers is held together by weak van der Waals interactions, which allow mechanical exfoliation of the WS2 layer. In the bulk phase, polymorphism is a unique feature of TMDs.
Properties of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Sunday, 11 September 2022 22:38
- Written by Caodan
- Hits: 1147
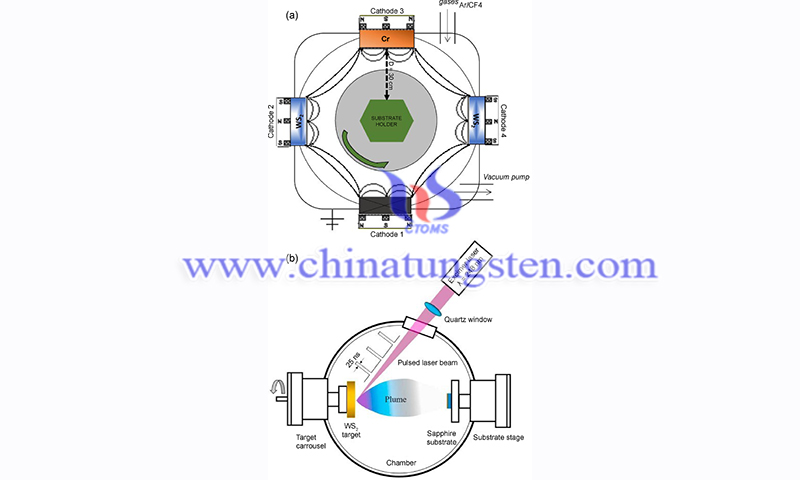
Owing to unique physical and chemical properties, transition metal dichalcogenides (TMDs) attract research interest. Among the family of TMDs, tungsten disulfide (WS2) has a unique band structure due to its semiconductor properties; i.e., its broadband spectral response characteristics, ultra-fast bleaching recovery time, and excellent saturable light absorption.
The 3 Most Common Tungsten Alloys—Its Properties & Applications
- Details
- Category: Tungsten Information
- Published on Tuesday, 06 September 2022 10:29
- Written by yuntao
- Hits: 1389

Alloys are metals made by combining two or more metallic elements, primarily to provide greater strength or corrosion resistance. The tungsten alloy family has many industrial applications due to its strength. Tungsten offers a unique contribution because it imparts exceptional strength, corrosion resistance and other useful properties to base metals. In addition to being an excellent alloying element, tungsten can also serve as the basis for its own alloys, and this article will focus on the basic categories of these tungsten alloys. Below are some details on the 3 most common tungsten alloys widely used in industry. Their properties and applications will also be introduced.
Read more: The 3 Most Common Tungsten Alloys—Its Properties & Applications





 sales@chinatungsten.com
sales@chinatungsten.com