Application of Tungsten Compounds to an Inhibitor against Bacterial Damage of Concrete
- Details
- Category: Tungsten's News
- Published on Tuesday, 26 April 2016 18:27


Atsunori NEGISHI, Terunobu MAEDA
Hazama Corporation.
Technical Research Institute.
Abstract
A series of tungsten compounds was tested to see the inhibiting effects against the growth of sulfuric acid producing bacteria (sulfur oxidizing bacteria; SOB) living in sewage treatment facilities and find out the way of eliminating the bad influence of sulfuric acidwhich attacks cement , penetrates into concrete , corrodes reinforcement and finally destroys the structures. Specimens of portland cement with or without tungsten compounds wereplaced in sewerage facilities and exposed to sewage. After 2 years of exposure, the compounds were found to have anti-bacterial effects.
Introduction
Recently, in Japan concrete structures have been corroded by sulfuric acid which has been produced by sulfur oxidizing bacteria in a short period of time1). The depth of corrosion has extended over 30mm in 10 years. In sewage, sulfate reducing bacteria would produce hydrogen sulfide from sulfate which is soluble in sewage2). When the flow of sewage is not turbulent, hydrogen sulfide is soluble in sewage, but when the flow is much turbulent byslopes or falls, hydrogen sulfide becomes gaseous. On concrete surfaces, sulfur oxidizing bacteria changes hydrogen sulfide into sulfuric acid and it reacts with alkali in the cement of the concrete and as corroded minerals, gypsum (CaSO4・H2O) will be produced. Because the mineral is very brittle, its surface can be easily peeled off by fingering3).
A conventional protection method against above corrosion has been mainly done by coating the surface of concrete, however it had such shortcomings that in several years the coating has expanded and peeled off because of its weak adhesion strength and permeated hydrogen sulfide gas4).
Maeda, et al. found that the growth of some sulfur oxidizing bacteria (SOB) called Thiobatillus thiooxidans was inhibited by nickel compounds5) , 6). However in lower pH (2-3), the bacteria did not stopped the growth completely, although in neutral pH (6-8) the bacteria stopped their growth. In lower pH, tungsten compounds were found to be able to inhibit the growth of the bacteria in place of nickel7). Tungsten is well known for its tensile properties, but application to an inhibitor against the growth of microorganisms was not noticed. Tungsten belongs to heavy metals, therefore, a toxicity to microorganisms could be expected although it shows generally low toxicity to human body compared to other metals8).
This report describes a study on the ability of tungsten compounds mixed in concrete as an inhibitor against SOB by exposing them in various conditions of sewage.
Materials and method
1. Inhibitors
Components of inhibitors admixed with mortar specimens were nickel or/and tungsten. Nickel compounds showed a good ability of inhibiting the growth of SOB at pH>7 while they didn’t inhibit well in the acidic state of pH<6. To compare the effect of these compounds, specimens were admixed with only nickel compounds, both nickel and tungsten, only tungsten and none of them respectively.
2. Test pieces
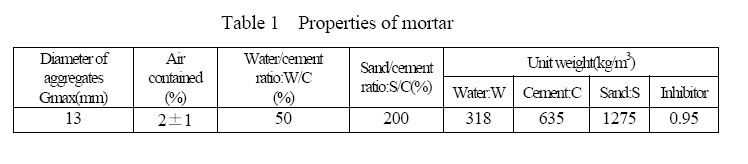
Mortar bars were used for the exposure tests. The sizes are all 40X40X160mm. Table-1 shows the mortar properties. The quantities of the inhibitors were decided by biological tests held in a laboratory. In the previous test, admixed nickel compounds as the inhibitor in the mortar were 0.2% of cement weight. In the exposure tests of this time, nickel and tungsten compounds were 0.075% for each, totaling 0.15% of cement weight. Test pieces were provided by a mortar mixer with the method of JIS A 11329). After 28 days of standard curing, they were exposed to sewage.
3. Exposure tests
Specimens were exposed to sewage in three different sewerage facilities shown below, separately located in Japan.
(1) Primary sedimentation tank of sewerage A which flowed hot spring water in the temperature of 30-40℃. The concentration of hydrogen sulfide was around 100ppm.
(2) Distribution tank of sewerage B in tropical region of Japan. Temperature was above 20℃ throughout the year.
(3) Distribution tank of sewerage C close to an industrial district. High COD(Chemical Oxygen Demand) sewage was flowing.
The surfaces of the concrete structures of all sites had been already deteriorated severely at the time of the tests.
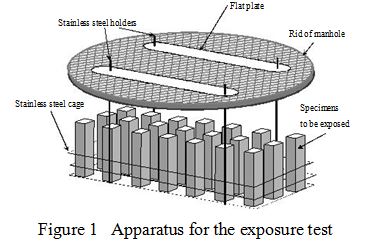
Figure 1 shows the method of an exposure test. Mortar bar specimens were placed vertically in a cage made from stainless steel wires. The cage was hung under a lid of a manhole at each exposure site and exposed to sewage. At regular intervals the specimens were taken out of the cage and examined to estimate the grade of corrosion. The analyses were made at each time 6 months, a year and 2 years later from the start of exposure tests respectively.
4. Analysis of weight loss
After the treatment of the surfaces of the specimens, they were moistened in standard curing tank for 48 hours. Then weight loss was measured with a balance. Corrosion grade was estimated comparing the weight losses of three specimens during exposure tests.
5. Analysis of depth
Gypsum (calcium sulfate hydrate : CaSO4・2H2O) was produced by SOB as a corroded material. It easily peeled off under the slightest shock. Proceedings of the corrosion were estimated precisely by measuring the depth of the corrosion with a micro measure. Three sides of the square on the specimens were measured on the direction of long axis. The depth was estimated by taking an average of three values.
6. EPMA analysis
The corroded materials were mineral substances mainly composed of calcium and sulfur, and produced by the reaction of sulfuric acid and cement composites. Gypsum which was one of the minerals resulting from the corrosion was a compound of calcium oxide and sulfate. Ettringite was that of calcium oxide, sulfate and aluminum oxide. The ratio of calcium to sulfur (Ca/S) was 1.0 for gypsum and 0.5 for ettringite. Nonaka, et al. described that sulfate penetrated into concrete of sewerage treatment facilities and there was secondary ettringite produced in front of the sulfate. The secondary ettringite produced by reaction with penetrated sulfate was different from primary ettringite produced at the time of setting fresh concrete. It became desirable that the grade of the corrosion could be estimated by measuring the distribution of these minerals in corroded concrete.
EPMA is an analytical instrument that can measure the distribution of elements in specimens. The distribution could be displayed in two-dimensional color mapping. When an electron beam which is accelerated to 15kV is irradiated to the specimens, characteristic X-rays of the elements in the specimens will be radiated. A count of the X-rays is proportional to the concentration of the elements, therefore their quantitative analysis is possible by comparing their counts with that of standard substances whose proportion is known exactly. The standard which was used in this study was a pure CaSO4・2H2O pellet made in fine crystals.
A sample was to be sliced off the square face (40x40mm) of the specimen in approximately 10 mm thick with a diamond cutter. The sliced surface was finished to make a shining one. Then it was coated with carbon membrane by a vacuum evaporator to make a conductor which can protect the specimen from being electrified by an electron beam during EPMA and render the measurement correct.
Results and discussion
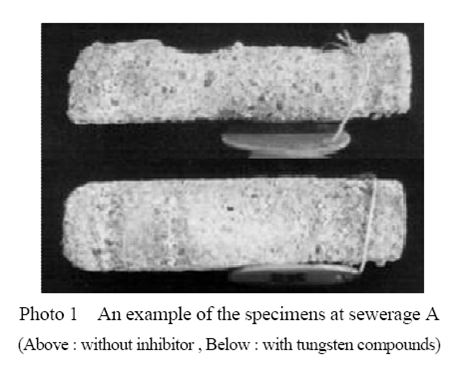
Photo 1 shows an example of the specimens after two years of exposure at three sites. Specimens mixed without inhibitors had completely white, brittle and rough surfaces at all sites, while specimens with inhibitors had almost smooth surfaces except ones at sewerage
A. The white substance on the surfaces of the corroded specimens had gypsum formation.
1. Weight loss
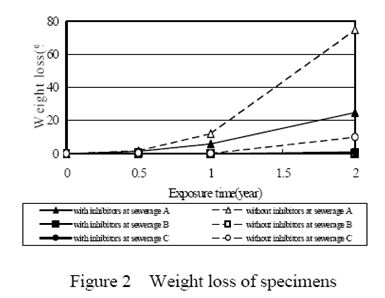
Figure 2 shows weight loss at every site. Weight loss of the contrast sample was bigger than the specimens with inhibitors. At sewerage A, the weight loss of the contrast sample was 37%/year and one of the specimens mixed with nickel-based inhibitor was 11%/year and that mixed with both nickel and tungsten compounds was 11%/year. At sewerage B, the weight loss of contrast sample was 2%/year and one of the specimens mixed with inhibitor of nickel compounds was 0.4%/year and that mixed with both nickel and tungsten compounds was 0.1%/year. At sewerage C, the weight loss of the contrast sample was 5%/year and one of the specimens mixed with inhibitor of nickel compounds was 3%/year and that mixed with both nickel and tungsten compounds was 0.5%/year. The weight loss of the specimens at sewerage A was the biggest among all sites because of its severest environmental conditions such as temperature, moisture and concentration of hydrogen sulfide.
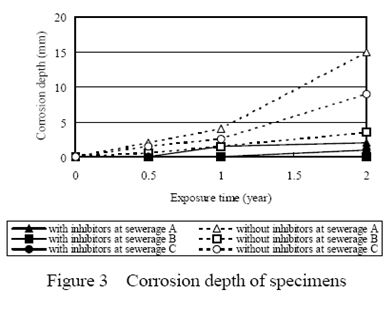
2.Corrosion depth
Figure 3 shows corrosion depth at every site. At sewerage A, the corrosion rate of the contrast sample was 7mm/year and one of the specimens mixed with nickel-based inhibitor was 1.5mm/year and that mixed with both nickel and tungsten compounds was 1.0mm/year. At sewerage B, the corrosion rate of the contrast sample was 2mm/year and one of the specimens mixed with inhibitor of nickel compounds was 0.4mm/year and that mixed with both nickel and tungsten compounds was 0.1mm/year. At sewerage C, the corrosion rate of contrast sample was 4.5mm/year and one of the specimens mixed with inhibitor of nickel compounds was 1.0mm/year and that mixed with both nickel and tungsten compounds was 0.7mm/year. The corrosion rate of the specimens at sewerage A was the biggest among all sites because of its severest environmental conditions such as temperature, moisture and concentration of hydrogen sulfide.
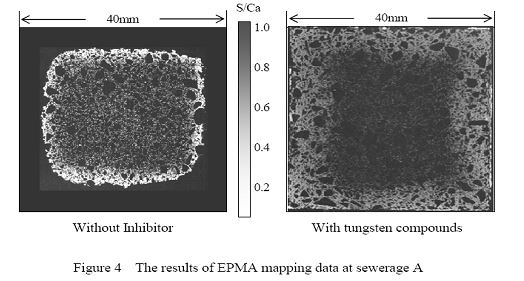
3.EPMA analysis
Figure 4 shows the results of EPMA mapping data of calcium and sulfur at each of the exposure site after 2 years exposure. A gray scale shows the elemental ratios of calcium to sulfur. As for the depth of sulfate penetrations into the specimen of sewerage A, in the case of specimen without inhibitors was around 10.0mm and that mixed with nickel compounds was 2.0mm and with tungsten compounds was 1.2mm. In the contrast sample at sewerage A, gypsum extended approximately 4.0mm in the specimen and in 4.0-10.0mm of depths, ettringite mainly existed. At sewerage B, the depth of sulfur penetration into specimens without inhibitors was 3.0mm and that with nickel compounds was 1.0mm and that with tungsten compounds was 0.5mm. At sewerage C, the depth of sulfur penetration into specimens without inhibitors was 6.0mm, and that with nickel compounds was 2.0mm and that with tungsten compounds was 1.0mm.
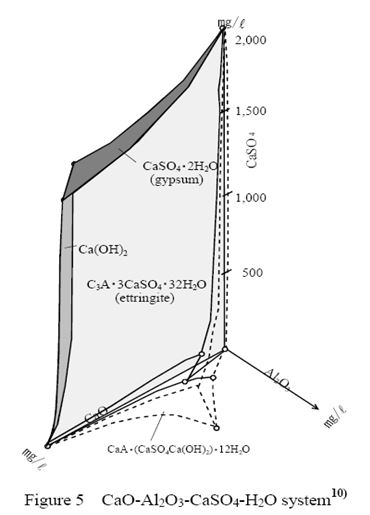
Figure 5 shows the equilibrium of CaO-Al2O3-CaSO4-H2O system. In the CaO-Al2O3-CaSO4-H2O system, if supply of sulfate to the specimens continues and the pH-value of the surface indicates around 2.0-4.0, gypsum would be produced in the system prior to ettringite. When the pH-value is above 4.0 and aluminates exist, ettringite would be formed prior in the system. Because the pH-value in ordinary concrete is about 12.0-13.0 and contains much aluminate, ettringite is the most stable mineral in the system. Sulfate in cement reacted with calcium aluminate during the setting of cement, because ettringite gave the primary strength to hardened concrete. Ettringite would be formed as a result of this reaction. But otherwise if the sulfate is to be contained over the criterion (3.0 % of cement weight in Japan) , ettringite would decrease the strength of concrete because of its expansibility. The layer of gypsum in the deteriorated concrete would be broken easily on the border of ettringite layer.
At sewerage A and sewerage C, composition of corroded materials observed with EPMA in the specimens without inhibitors were almost gypsum and a few ettringite. In this site, the rate of producing sulfuric acid of SOB was faster than the rate of supplying alkali of inner concrete. Existence of ettringite was only observed on the surface of the specimens with inhibitors. This would suggest that inhibitors prevent SOB from producing sulfuric acid.
At sewerage B, corroded mineral observed in EPMA analysis on the specimens without inhibitors was only ettringite and not gypsum. SOB produced sulfuric acid finally, and the acid reacted with calcium hydroxide at the time of setting the cement. On the surface of the specimen, gypsum reacted immediately with aluminate, and alkali was supplied from inner part of the specimen and changed into ettringite, because in the equilibrium of the CaO-Al2O3-CaSO4-H2O system the supplying rate of aluminate and alkali was faster than the formation rate of sulfuric acid. It was found that ettringite was formed in a wide range of concentration of CaO, Al2O3 and CaSO4. The system was observed at the time of setting the cement, therefore it would be possible to apply the system which exists in millipore solution of hardened concrete.
No corroded mineral was observed on the surface of specimens with inhibitors because inhibitors prevented SOB from producing sulfuric acid.
Conclusion
Based on the exposure tests, the following conclusion was obtained.
(1) Tungsten compounds were available as inhibitors against SOB, considering from the result of the comparison tests to specimens without inhibitors.
(2) The inhibiting ability of tungsten compounds was stronger than nickel compounds in the severe deterioration at sewerage A.
(3) Inhibiting ability of tungsten compounds against Thiobatillus thiooxidans worked well in lower pH (pH<3).
(4) Admixing of both nickel and tungsten compounds to concrete would enhance the inhibiting ability of concrete within a wide range of pH value.
Acknowledgment
The authors wish to thank Prof. Sugio of Okayama University who cooperated in the progress of biological tests, and also appreciate the cooperation of managers in three sewerage facilities through the exposure tests.
References
(1) Mori,T. et al.,‘Interaction of nutrients, moisture and pH on microbial corrosion of concrete sewer pipe’, Wat. Res. 26(1)(1992) 29-37
(2) Nonaka, T., Yang, W., Fujisawa, K., and Tamura, H., ‘Hydrogen sulfide generation in wastewater treatment plants in microbial corrosion of concrete structure in Japan’, Shimane University (1997) 111-119.
(3) ‘Anti-deterioration technique of concrete structure of sewerage’, Japan Sewerage Works Agency, Hazama Co. and Nippon hume Co.(1998) 1-7
(4) Okamoto, S., et al., ‘Development of economical methods to protect hydrogen sulfide at sewerage’, Public Works Research Institute , Hazama Co. and et al.,(1998) 90-99
(5) Terunobu maeda, Atsunori Negishi, in “Fracture and Damage of Concrete and Rock-FDCR-2,”ed.by H.P.Rossmanith,E&FNSpon,(1993) 307-314
(6) Yasuo Nogami, Terunobu maeda, Atsunori Negishi, and Tsuyosi Sugio, ‘Inhibition of sulfur oxidizing activity by nickel Ion in Thiobacillus thiooxidans NB1-3 isolated from the corroded concrete’ , Biosci.Biotech.Biochem.,61(8), (1997) 1373-1375
(7) Terunobu maeda, ‘Studies on the development of bacteriostatic agents for the prevention of concrete corrosion’, a doctoral thesis at Okayama University (1999)
(8) ‘Material safety data sheet’ ,Tungsten powder, Hummel Croton Inc.(1996)
(9) ‘Method of Making and Curing Concrete Specimens’, JIS A 1132(1976)
(10) D’Ans, J., et al., ‘The system CaO-Al2O3-CaSO4-H2O at 20 oC’, Z.K.G.,6,(1953) 304
Article source from: '8th International Tungsten Symposium in Fukuoka Japan ,1999'
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com