Replacing Lead Oxide with Tungsten Trioxide Improves the Performance of Photovoltaic Front Silver Paste
- Details
- Category: Tungsten Information
- Published on Wednesday, 01 June 2022 14:00

The silver paste for the front electrode of solar cells is one of the main raw materials that affect the electrical properties of solar cells. The photovoltaic front silver paste is mainly composed of three parts: conductive phase, organic term and inorganic term.
Synthesis and Characterization of Molybdenum Carbide Catalysts on Different Carbon Supports
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2022 22:51

The synthesis and characterization of molybdenum carbide catalysts on different carbon supports were investigated by researchers from Henan Polytechnic University. Molybdenum carbide was prepared by carburization of ammonium molybdate on cellulose, g-C3N4, MWCNTs, and sodium lignosulfonate without using any gaseous carbon source. In addition, the formation of molybdenum carbide was investigated in depth by in situ characterization using a simultaneous thermal analyzer-quadrupole mass spectrometry system.
Nucleation and Epitaxy of Bilayer Molybdenum Disulfide on Sapphire
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2022 22:47
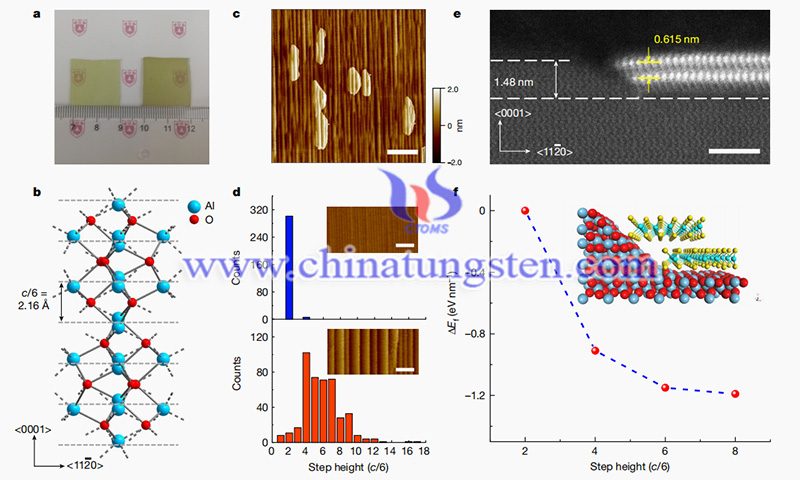
A study conducted mainly by researchers at Nanjing University reports the uniform nucleation (>99%) of bilayer molybdenum disulfide (MoS2) on c-plane sapphire. Two-dimensional transition-metal dichalcogenides (TMDs) are of interest to researchers in the field of electronics. It is believed that the combination of good electrostatic control, smaller bandgap, and higher mobility than single-layer TMDs has the potential to improve the power-delay products of transistors. However, despite the progress made in the growth of single-layer TMDs, multilayer controlled epitaxial growth remains a challenge.
Biochar-Based Molybdenum Fertilizer Enhances N Assimilation in Cabbage
- Details
- Category: Tungsten Information
- Published on Monday, 02 May 2022 11:52

Low molybdenum (Mo) bioavailability in acidic soils hinders N assimilation by vegetables, and providing available Mo in acidic soils is a challenge to reduce nitrate accumulation in vegetables. A study conducted by the Institute of Guangdong Academy of Agricultural Sciences investigated three methods of molybdenum application: biochar-based molybdenum fertilizer (Mo-biochar), seed dressing, and basal application to improve Mo bioavailability and N assimilation in cabbage (Brassica parachinensis) in acidic soils.
A Novel Model for Band Structure of Monolayer Molybdenum Disulfide
- Details
- Category: Tungsten Information
- Published on Monday, 02 May 2022 11:47
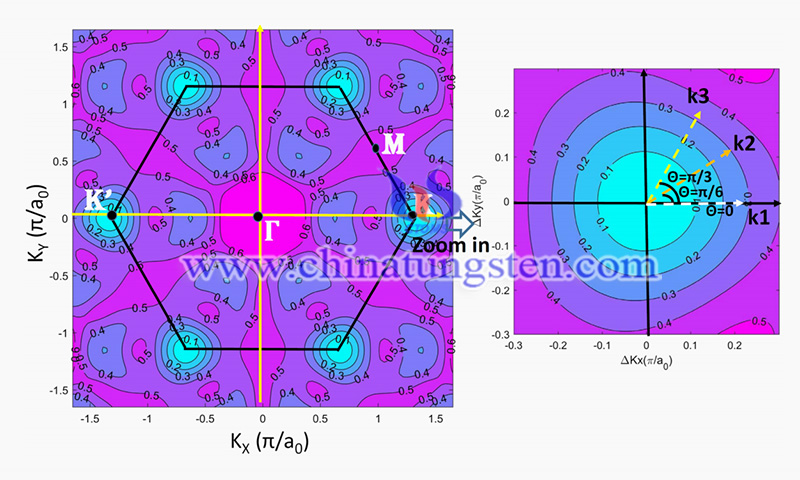
Recently, researchers have proposed an analytical band calculation (ABC) model to study the band structure of monolayer molybdenum disulfide. For two-dimensional (2D) materials, a semiconductor layer is about the thickness of an atomic layer. 2D materials are gaining attention for their potential applications in future transistor manufacturing processes. 2020 International Roadmap for Devices and Systems (IRDS) predicts that by 2028, 2D materials will be the optimal choice for channel material technologies and CMOS 2D device applications.
Nano Tungsten Trioxide in Lithium Ion Battery
- Details
- Category: Tungsten Information
- Published on Wednesday, 27 April 2022 15:25

As one of the recent hot topics, cobalt-free batteries can simply be considered as an upgraded version of the current commercial ternary lithium batteries. Because of their higher energy density and lower production costs, they are popular among many battery manufacturers. So, as a typical transition metal N-type semiconductor material, how is tungsten trioxide used in cobalt-free lithium ion battery?
Tuning Electronic Structure of Tungsten Oxide for Hydrogen Electrolyte Reaction
- Details
- Category: Tungsten Information
- Published on Friday, 15 April 2022 12:32
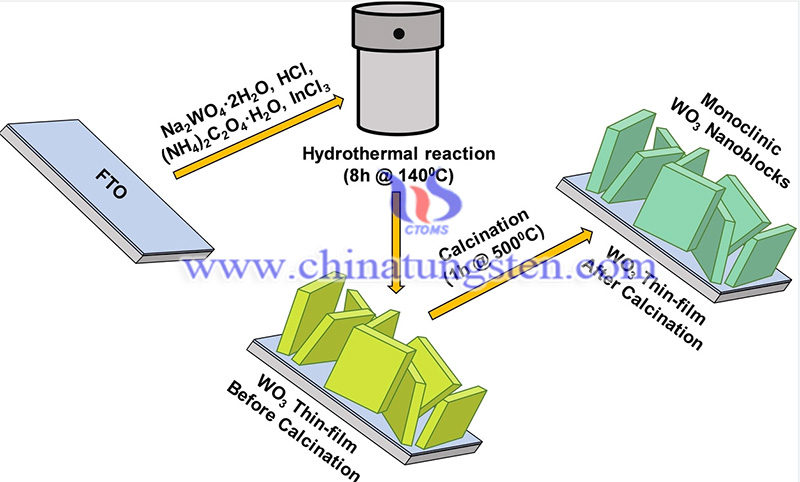
Researchers at the Shanghai Institute of Microsystems and Information Technology (SIMIT), Chinese Academy of Sciences (CAS) have demonstrated that doping tungsten oxide (WO3) catalysts with appropriate cations can change the interfacial structure, surface chemical state, and bandgap values, thus improving the performance of the basic hydrogen electrolyte reaction (HER). In the experiments, cation (Ni, Co, and Fe) doped WO3 catalysts were synthesized on nickel foam. The Co-doped catalysts (Co WO) exhibited remarkable HER activity.
Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials
- Details
- Category: Tungsten Information
- Published on Friday, 15 April 2022 12:27
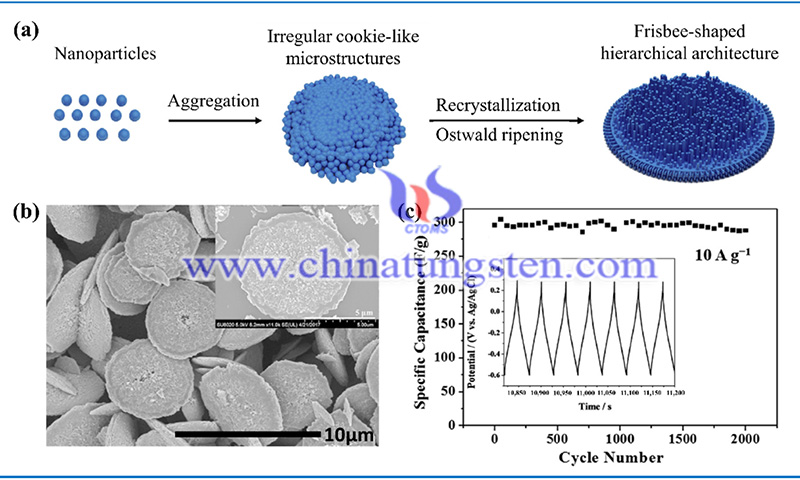
Tungsten oxide-based nanomaterials have attracted a lot of attention for their use in building various electrochemical energy devices. In particular, electrochromic devices and optical change devices have been intensively investigated in terms of energy conservation.
Tungsten Oxide Polymorphs and Applications
- Details
- Category: Tungsten Information
- Published on Tuesday, 12 April 2022 11:40
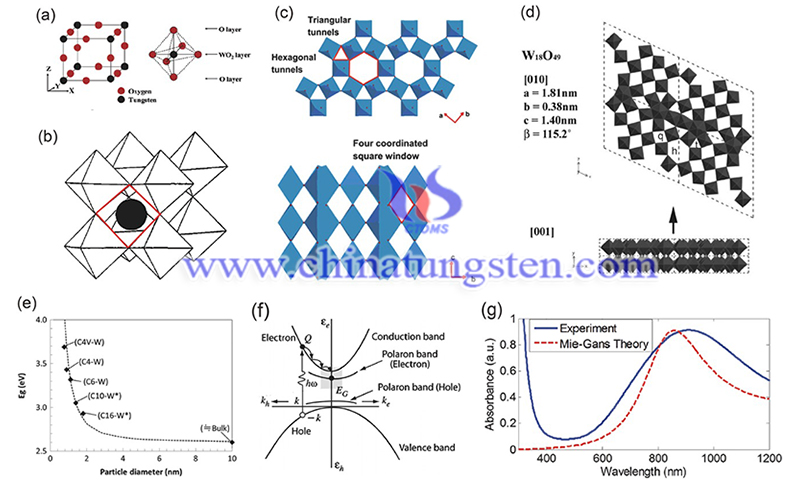
Considerable interest in tungsten oxide polymorphs (WO3-x) nanomaterials has been generated due to their abundance in nature, easy availability, high stability, non-recombination, and chemical diversity, and many advances have been made from traditional catalysts and electronics to emerging artificial intelligence. A recent study from Qingdao University presents the latest progress of WO3-x polycrystals and their multifunctional applications.
Oxidation Protection of Tungsten Alloys in Nuclear Fusion Applications
- Details
- Category: Tungsten Information
- Published on Tuesday, 12 April 2022 11:37
A recent study conducted by the Anhui University of Technology has proposed new measures for the oxidation protection of tungsten alloys in nuclear fusion applications. As a plasma guide material (PFM), tungsten is persistently used in nuclear fusion reactors. However, it has poor oxidation resistance at high temperatures. When a reactor de-cooling accident occurs, tungsten is rapidly oxidized and volatilized due to air entering the vacuum chamber, which may cause a catastrophic nuclear leakage accident.



 sales@chinatungsten.com
sales@chinatungsten.com