Oxidation Behavior of W-Cr Binary Alloys
- Details
- Category: Tungsten's News
- Published on Friday, 21 April 2023 22:11
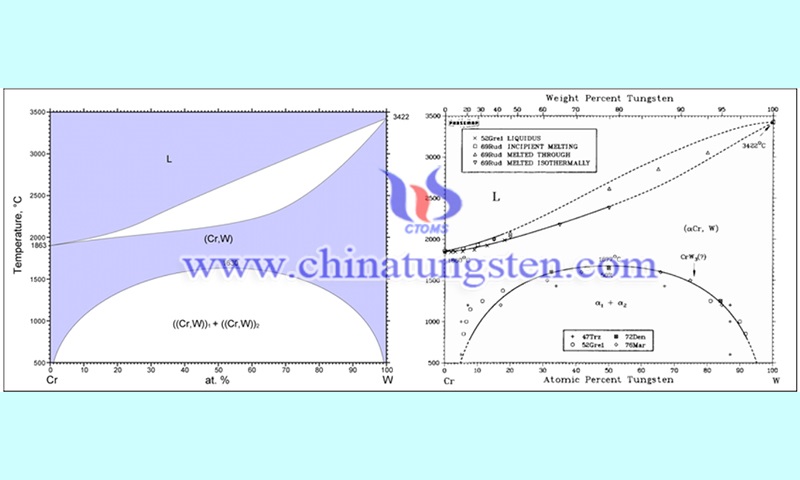
Cr is a traditional antioxidant beneficial element that is widely used in the oxidation protection process of metals. In addition, it has the same body-centered cubic structure as the tungsten element. Therefore, the oxidation behavior of W-Cr binary alloys has been studied extensively by related scholars. Telu et al. prepared W0.7Cr0.3, W0.5Cr0.5 and W0.4Cr0.6 alloys by mechanical alloying (MA) and observed that the two phases (Wss, Crss) have significantly different brightness.
(Photo credit: Jan Cizek / coatings)
Researchers investigated on the increase in mass per unit area (Δm/S) of the alloy in air at 1000°C and 1200°C. The Δm/S of the alloy was 0.2 to 0.5 times that of pure W during the first 5 hours of exposure at 1000°C. The relatively high Δm/S value of the W0.7Cr0.3 alloy compared to other binary alloys is a limitation to improve the oxidation resistance of pure W. Similar conclusions were obtained in the oxidation experiments at 1200°C. This is due to the relatively low content of Cr elements in the W0.7Cr0.3 alloy, which cannot form a dense Cr2O3 protective film on its surface during oxidation.
However, the mass gain of the W0.5Cr0.5 alloy was the lowest in the above experiments. This indicates that there is an upper limit to the increase of Cr element content for improving the oxidation resistance of the W-Cr binary alloys. A more uniform and fine Cr-rich phase was observed on the surface of the alloy. This facilitates the formation of a continuous and dense oxide film on the surface of the alloy at the initial stage of oxidation. The EDS and XRD results show that the oxide layer consists of Cr2O3, Cr2WO6 and porous WO3 layers from the inside to the outside.
In the initial stage of oxidation, W and Cr are oxidized simultaneously on the alloy surface, and Cr2O3 and WO3 grow along the alloy surface. In addition, the volume expansion of WO3 increases the porosity and the thickness of the oxide layer as the reaction proceeds. Due to the relatively low oxygen partial pressure in the oxide layer, the Cr element is greatly oxidized. Finally, a continuous Cr2O3 layer was formed at the bottom of the oxide layer. Furthermore, similar conclusions were obtained by Hou et al. researchers when they studied the oxidation behavior of W-Cr (10, 20 wt% Cr) alloys.

(Photo credit: Jan Cizek / coatings)
Reference: Fu T, Cui K, Zhang Y, et al. Oxidation protection of tungsten alloys for nuclear fusion applications: A comprehensive review[J]. Journal of Alloys and Compounds, 2021, 884: 161057.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com