SMENA Catalysis AB Develops MoS2 Material for Gas Sensing and Hydrogen Catalysis
- Details
- Category: Tungsten's News
- Published on Saturday, 03 December 2022 19:10
Research by the Swedish company SMENA Catalysis AB has led to the discovery of a MoS2 material, a method for improving the properties of layered molybdenum disulfide (MoS2) by introducing highly controllable edges and holes in the material.
Timur Shegai and Batulga Munkhbat's research on transition metal dichalcogenides (TMDs) led to the creation of SMENA Catalysis AB in 2021. Affiliated members of this graphene flagship program are spin-off companies from Chalmers University of Technology, Sweden, a partner of the Graphene Flagship Program.
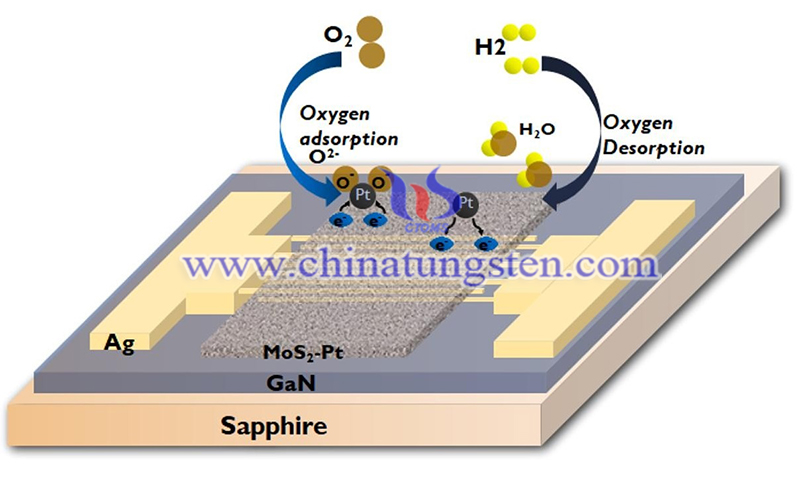
(Credit: Sandeep ReddyGottam et al., Applied Surface Science)
SMENA Catalysis AB was founded with the support of Chalmers Innovation Office and Chalmers Venture Partners. After the first CEO was appointed, the company was quickly accepted into the award-winning Chalmers Venture Accelerator program and received its first seed funding as well as administrative and business development support. The company has now expanded its workforce and launched its first collaboration with industry players.
SMENA Catalysis AB has two business units, Gas Sensors and Hydrogen Catalysis, which use the processed MoS2 material Molybdenyx. For gas sensors, the company supplies customers with gas sensing elements, which can be thought of as small microchips. The chemical reaction is read by a detector, which decodes the concentration and type of gas.
The Hydrogen Catalysis division provides Molybdenyx in powder form that can be applied to a proton exchange membrane (PEM)-electrolyzer to facilitate the chemical reaction (electrolysis) that breaks down water into hydrogen and oxygen and produces hydrogen gas.
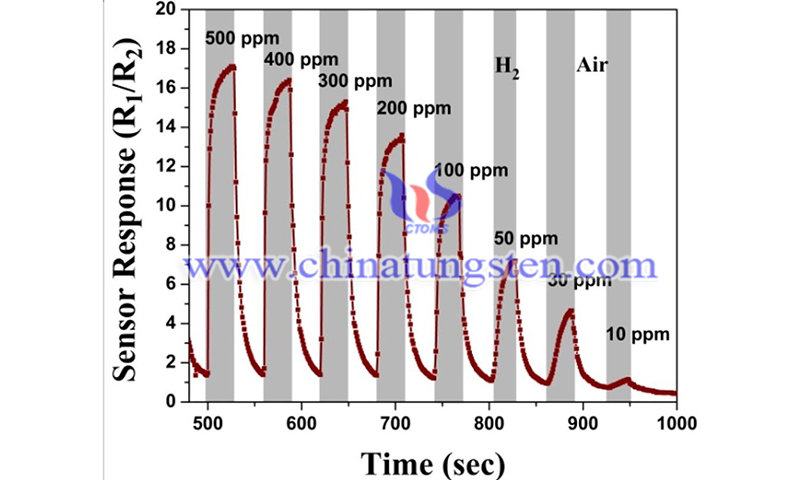
(Credit: Sandeep ReddyGottam et al., Applied Surface Science)
In gas sensing, metal oxide and carbon nanomaterial-based gas sensors are hampered by high temperature requirements, high power consumption, high sensitivity to changes in humidity, low sensitivity and incomplete recovery at room temperature. Metal nanoparticle sensors can be "poisoned" by gas molecules, reducing their ability to detect target molecules.
Conductive polymers can operate at room temperature, but they are highly sensitive to humidity. SMENA Catalysis AB edge-enriched MoS2 materials contain a large number of active sites, i.e. their materials have a large number of hexagonal pores. These active sites absorb gas molecules with higher sensitivity, interact more specifically with selected gases and are unaffected by changes in humidity. This allows benefits that could not be combined before, such as sensitivity, selectivity, accuracy and low energy consumption, to be combined in a tiny sensor.
MoS2 is also an abundant, naturally occurring mineral that is environmentally sustainable and economically profitable. The breakthrough in this technology is to make holes in the material and then expose these holes to a liquid solution. Depending on how long the prefabricated holes are exposed to this liquid solution, these holes will get closer and closer to our unique hexagonal shape.
SMENA's target customers are gas sensor companies that offer or wish to expand their portfolio to MOS-gas sensors capable of detecting low concentrations of H2, NH3 and NO2. Currently SMENA Catalysis focuses on manufacturers of PEM-electrolyzers and membrane electrode assemblies (MEAs).
References: Munkhbat, Battulga, et al. "Transition metal dichalcogenide metamaterials with atomic precision." Nature communications 11.1 (2020): 1-8.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com