How Tungsten Oxide Can Be Used as Catalyst in Chemical Transformations
- Details
- Category: Tungsten's News
- Published on Friday, 20 May 2022 20:11
A new study has recently shown us how tungsten oxide (WO3) can be used as a catalyst in sustainable chemical transformations. The project uses computational simulations to understand how WO3 interacts with hydrogen at the molecular level and validates these findings through laboratory experimentation.
Engineers rely on catalysts for a wide range of applications, from food manufacturing to chemical production, so the search for efficient, environmentally friendly catalysts is an important research direction. New research led by the University of Pittsburgh Swanson School of Engineering may lead the way to new sustainable catalysts based on WO3 and similar compounds.
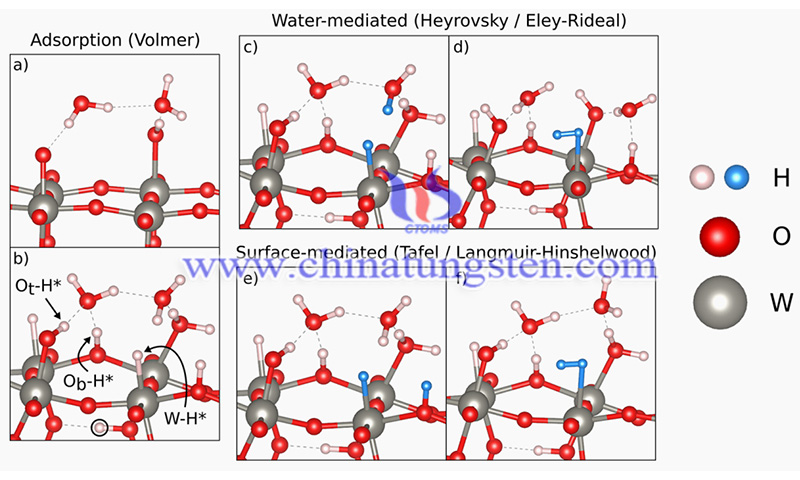
(Credit: Evan V. Miu/JACS)
The study titled “The Sensitivity of Metal Oxide Electrocatalysis to Bulk Hydrogen Intercalation: Hydrogen Evolution on Tungsten Oxide.” has been published in the Journal of the American Chemical Society (2022) 144 (14). The study was carried out by Evan V. Miu, James R. McKone, and Giannis Mpourmpaki.
"WO3 is a catalyst that can accelerate sustainable chemical conversion through the use of sunlight or renewable electricity." Mpourmpakis said, "This compound has a unique way of interacting with hydrogen atoms that makes it particularly adept at participating in chemical reactions that require the production or use of hydrogen."
"The types of chemical reactions we are most interested in include the use of hydrogen to convert carbon dioxide into useful fuels and chemicals," McKone added.
While most catalysts only interact with molecules such as hydrogen on their surfaces, tungsten oxide can also insert hydrogen into its three-dimensional lattice. The researchers' advanced modeling was able to show that this process has a huge impact on what actually happens at the catalyst surface.
This work opens up the possibility of designing a whole new family of catalysts based on tungsten oxide and similar compounds, using the team's computational approach to predict their catalytic properties. McKone said, "We can design catalysts that deliver hydrogen in just the right way to make the chemical conversion of water and electricity as efficient as the fossil fuels we use today."
This project is a collaboration between the CANELa lab at Mpourmpakis and the McKone lab, with lead author Miu, a graduate student at the National Science Foundation, investigating the bridge between thermal and electrocatalysis through the application of experimental and computational methods.
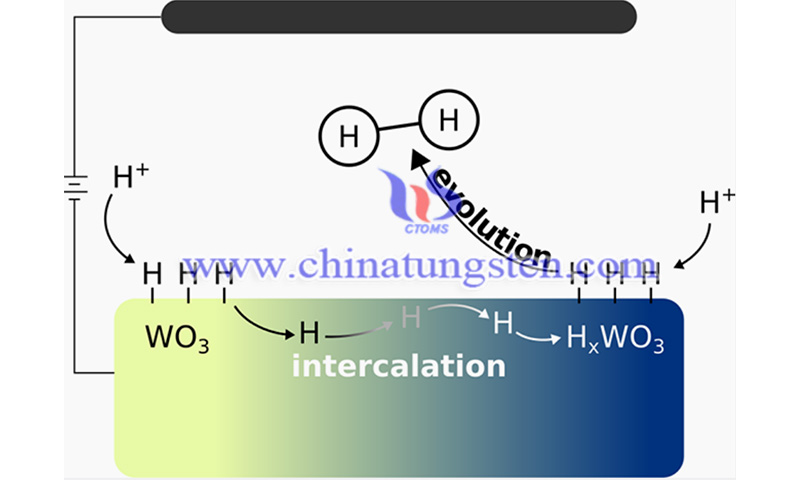
(Credit: Evan V. Miu/JACS)
Miu said, "Working with Mpourmpakis and Prof. McKone gave us the opportunity to operate at the interface of our experiments. These helped us gain insight into how metal oxide bronzers catalyze hydrogen, and we are excited to apply our findings and take a meaningful step toward more sustainable chemical processes."
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com