Path to Rare Earth Formation Effects Green Energy and Smart Tech
- Details
- Category: Tungsten's News
- Published on Sunday, 23 May 2021 10:09
Researchers at Trinity College Dublin have shed new light on the rare earth formation mechanism. The use of rare earths in green energy and smart tech industries is in increasing demand worldwide. Their discovery has important economic significance because there are no substitute alternatives for rare earth elements (REE), which are indispensable due to their ability to form small and very powerful magnets essential for the generation of smart devices and low-carbon energy.
Most REEs are mined in carbonate deposits, the largest known carbonatite is China's Bayan Obo, but due to its complex mineralogy, element composition and geological history, scientists are still debating about how and why they form.
There are more than 250 known rare earth-containing minerals, but only three are economically viable and can be exploited commercially. Bastnäsite may be the main valuable mineral of the global REEs, and is the focus of the Trinity team's research.
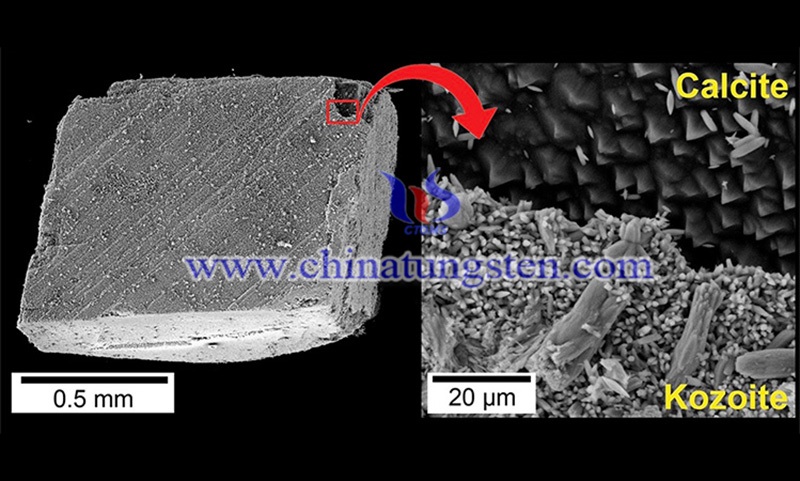
By considering the interaction between water containing rare earth elements and calcite, calcite is a mineral that is ubiquitous in nature and often present in hydrothermal environments. Therefore, the research team found a new way to form bastnäsite.
Dr. Adrienn Maria Szucs candidate of the Trinity is the first author of the study of rare earth formation, which has just been published by the international journal Crystal Growth & Design. She said: "The fact that we need more rare earth elements motivates us to learn more about the geochemical behavior of these precious elements. Simply put, we need to know a lot more about REEs, and how and why they form, if we want more of them".
"The crystallization pathways we discovered indicate that in some rare earth-bearing deposits, the origin of bastnäsite may simply be the result of the interaction between calcite and fluids rich in rare earths. This is not the only reaction to form bastnäsite, but this discovery is particularly important because calcite is everywhere and is the most stable calcium carbonate in nature. Therefore, this suggests that it should be possible to support the formation of magnesite under appropriate conditions."
Juan Diego Rodriguez-Blanco, Ussher Assistant Professor of Nanomineralogy at Trinity, and funded investigator of the Irish Centre for Research in Applied Geosciences (iCRAG), is the lead researcher. He said: "Over the years, the use of rare earth elements in high-tech products has been increasing, so the demand for them has is also shooting up. As many rare earth elements have become very valuable for green energy and smart tech, this has caused huge geopolitical competition.
"Unfortunately, the extraction and purification of rare earth elements are both economically and environmentally expensive. Therefore, the work of rare earth formation is very important for bettering our understanding of the formation mechanism of bastnäsite, which in turn helps us improve the existing extraction and refinement methods."
- Rare Earth Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com