MoS2 Structure Alleviates Shortcomings of 2D Materials in Battery Applications
- Details
- Category: Tungsten's News
- Published on Monday, 15 March 2021 20:51
Researchers from Tongji University and Fudan University have designed a new structure of molybdenum disulfide (MoS2), namely the covalently assembled superstructure between MoS2 layers, which effectively alleviates the shortcomings of dimensional materials in battery applications. It acts as a linkage for lithium/sodium‐ion batteries.
Weak van der Waals interactions between interlayers of two‐dimensional layered materials result in disabled across‐interlayer electron transfer and poor layered structural stability, seriously deteriorating their performance in energy applications. Herein, the study proposes a novel covalent assembly strategy for the nanosheets to realize unique MoS2/SnS hollow superassemblies (HSs) by using SnS nanodots as covalent linkages.
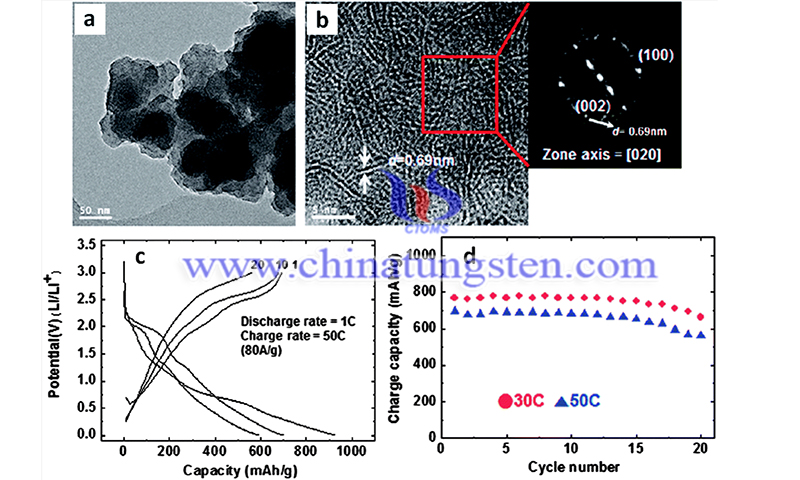
The covalent assembly based on all‐inorganic and carbon‐free concept enables effective across‐interlayer electron transfer, facilitated ion diffusion kinetics, and outstanding mechanical stability, which are evidenced by experimental characterization, DFT calculations, and mechanical simulations. Consequently, the HSs exhibit superb rate performance and long cycling stability in lithium‐ion batteries, representing the best comprehensive performance in carbon. Moreover, the HSs also show excellent sodium storage performance in sodium‐ion batteries.
Two-dimensional materials refer to materials in which electrons can only move freely (planar motion) on the nanometer scale (1-100nm) in two dimensions, such as nano-films, superlattices, and quantum wells. It is considered to be an ideal electrode material for a new generation of high-performance secondary ion batteries due to its larger interlayer spacing and higher theoretical energy storage capacity.
However, the typical weak van der Waals force between the layers of the material prevents electrons from being transported perpendicularly to the lamellae, which reduces the overall electron transport efficiency of the material. And the layered structure is also easily destroyed during the repeated deintercalation of ions, and eventually causes serious deterioration of battery performance.
Researchers from Tongji University and Fudan University took the typical layered material molybdenum disulfide as the research object, and by designing a new covalent assembly strategy, they constructed ultra-thin molybdenum disulfide nanosheets and stannous sulfide nanodots. The hollow superstructure assembled with covalent bonds is used in battery applications - high-performance lithium/sodium-ion batteries.
The specific advantages are as follows: First, stannous sulfide is uniformly anchored between the MoS2 lamellae in the form of nano-dots, which enhances the force between the lamellae and gives the material structure high mechanical stability. Second, the constructed interlayer covalent bond provides a channel for the vertical transmission of electrons between the layers, which improves the overall electron transmission efficiency of the material.
Third, DFT theoretical simulation calculations also show that the structure can reduce the lithium-ion diffusion and migration barrier, which is conducive to the rapid occurrence of electrochemical reactions. It is conducive to the improvement of battery rate performance. Fourth, through in-situ electron microscopy and mechanical calculation and analysis, the covalent assembly of superstructures between molybdenum disulfide layers can effectively alleviate the stress generated during lithium-ion insertion and keep the structure intact during cycling.
Fifth, the covalently assembled superstructure exhibits high specific capacity, excellent rate performance, and good cycle stability in lithium/sodium ion battery applications. The research results were published in Angewandte Chemie International Edition under the title of "Covalent Assembly of MoS2 Nanosheets with SnS Nanodots as Linkages for Lithium/Sodium-Ion Batteries".
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com