How to Make a Preparation of Tungsten Diiodide from Tungsten Hexachloride?
- Details
- Category: Tungsten Information
- Published on Tuesday, 27 June 2023 22:34
Tungsten hexachloride (WCl6), as one of the reactants, can be reacted with hydrogen iodide (HI) to prepare tungsten diiodide, but attention should be paid to the temperature of the reaction to reduce the chances of the formation of other products.
The reaction of tungsten hexachloride with hydrogen iodide is divided into two parts: halide substitution reaction and screening of the products. Since the element iodine has the smallest electronegativity among the halogen elements, the attraction of iodide ions to hydrogen ions is weak, so the number of free-moving hydrogen ions is more, and the reducibility and aqueous acidity of hydrogen iodide is stronger, which possesses the ability to replace chlorine of tungsten hexachloride and produce hydrogen chloride (HCl). At the same time, the weak attractive force also leads to the fact that hydrogen iodide is the least stable of the halogenated gaseous hydrides, both of which have low melting and boiling points of -50.8°C and -35.1°C, respectively. Therefore, the halide substitution reaction portion of the reaction requires a sealed vessel with the temperature set at -51°C to ensure that the hydrogen iodide is in the liquid state and does not give excessive gas pressure to the sealed vessel.
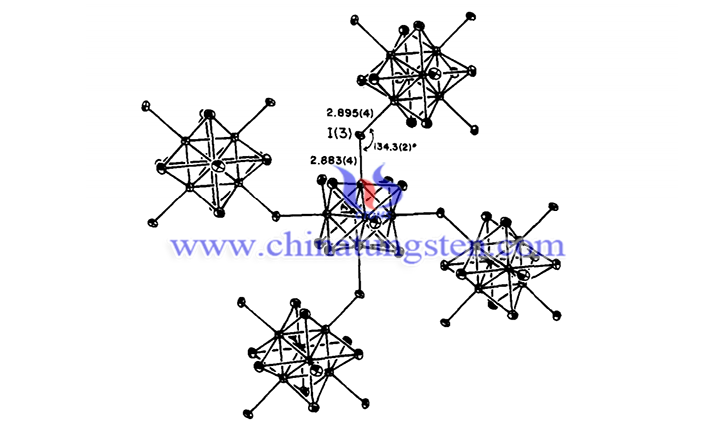
The products obtained after the completion of the reaction are mixtures and require further screening. In the past, a researcher heated the product to 110°C after the halide substitution reaction and obtained a final composition of tetraiodotungsten (WI4), which implies that the change of temperature has a great influence on the content of the final product.
Due to technological advances, the containers used for the reaction have been improved, the capacity of air pressure has been increased, and the emergence of reaction tubes has made high-temperature reactions more possible than the low-temperature reactions used in the preparation of tetraiodotungsten iodide. After continuous attempts by researchers, tungsten diiodide was finally obtained in a reaction tube at 400°C. Not only that, the reaction eliminated the process of liquefying hydrogen iodide, making the reaction a one-step process and giving more possibilities for the preparation of tungsten diiodide.
This method still has shortcomings, such as the difficulty of pilot scale-up of the reaction. Due to the instability of halide substitution, the exchange reaction may be incomplete, so the halide substitution reaction method is more suitable for small scale.

- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com