WO3–Pt/C Electrocatalysts for Oxygen Reduction Reaction
- Details
- Category: Tungsten Information
- Published on Monday, 13 September 2021 14:21
Oxygen reduction reaction (ORR) has been widely studied for its applications in fuel cells. There is a growing demand for clean energy technologies such as fuel cells. Since the energy efficiency and battery voltage of electrochemical cells are limited by the slow kinetics of ORR.Currently, platinum dispersed on carbon (Pt/C) is the most active catalyst for ORR; however, in addition to its high cost, this metal also has problems with ORR overpotential and poor methanol tolerance. Supporting by transition metal oxides such as tungsten trioxide (WO3) and titanium dioxide (TiO2) to the Pt/C electrocatalyst provide not only active sites but also electronic and ionic conductivity.
Therefore, WO3–Pt/C electrocatalysts has been synthesized by chemical reduction for oxygen reduction reaction, the result show higher kinetic activity for oxygen reduction reaction in a single cell than that of conventional Pt/C electrode. The preparation method of is as follows:
Use hexachloroplatinic acid (H2PtCl6·H2O) (Aldrich, ≥37.50% Pt basis) to prepare monometallic Pt/C catalyst by impregnation on Vulcan XC-72R carbon (Cabot Corporation), with a nominal platinum content of 10%wt Pt (Pt/C sample). The carbon was impregnated with a solution of platinum precursor and ethanol, and the mixture was stirred at room temperature for 1 hour. The product was then filtered and slowly dried at about 30°C. Finally, the sample was treated in hydrogen (99.95%) at 300 °C for 2 hours. WO3–Pt/C catalyst is prepared by chemical reduction of an ethyl solution of ammonium metatungstate (NH4)6H2W12O40 (Aldrich, 99.99%) at 70°C using a previously prepared Pt/C catalyst. Finally, the sample is washed, filtered and slowly dried. Two samples with nominal WO3 content of 5% and 10% wt (5% WO3-Pt/C and 10% WO3-Pt/C samples respectively) were prepared.
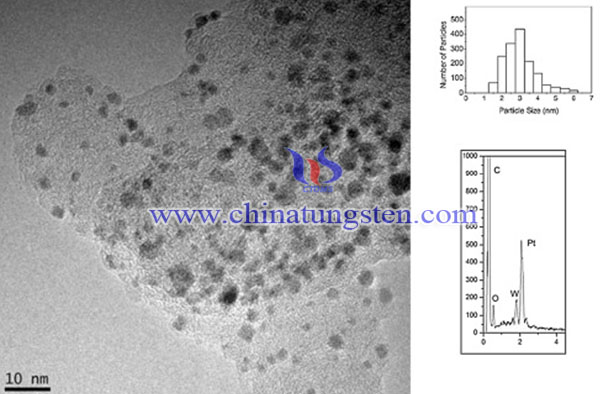
In summary, WO3–Pt/C electrocatalysts has been synthesized for oxygen reduction reaction, the result show higher kinetic activity for oxygen reduction reaction in a single cell than that of conventional Pt/C electrode. The open circuit voltage is approximately 0.94 V at operating temperature, and the maximum power density at 40 °C is 90 mW cm-2, which increases to 115 mW cm-2 at 80 °C. These values are higher than the power density obtained by the self-made Pt/C catalyst, indicating the promoting effect of WO3 nanostructures. This increase in electrocatalytic activity is attributed to the interaction between Pt and WO3, where WO3 nanostructures remove oxygen atoms from platinum and increase hydrogen spillover.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com