Chemical Methods for WS2 Film Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 September 2022 00:41
- Written by Caodan
- Hits: 1103
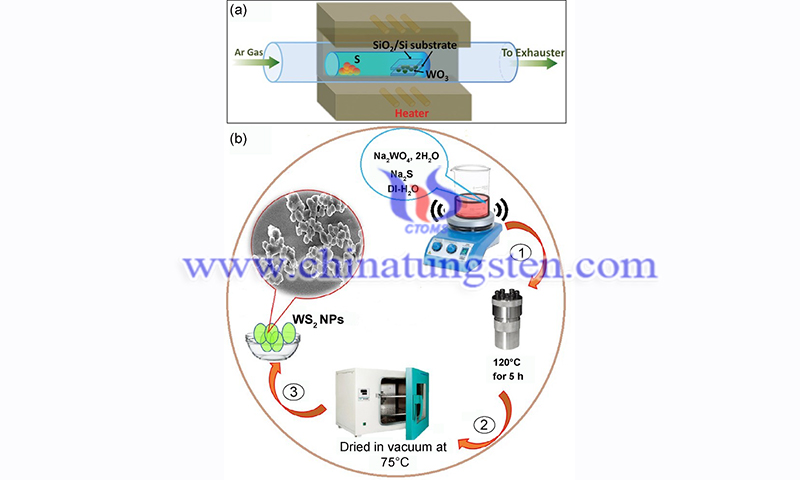
Two common methods for preparing tungsten disulfide (WS2) films by chemical methods are chemical vapor deposition (CVD) and hydrothermal growth of single-crystal tungsten disulfide from aqueous solutions under high temperature and pressure conditions. CVD is the most common method used to prepare tungsten disulfide. The CVD method involves a reaction process in which a gaseous precursor reacts chemically on a solid surface to produce a solid deposit.
Properties of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Sunday, 11 September 2022 22:38
- Written by Caodan
- Hits: 1039
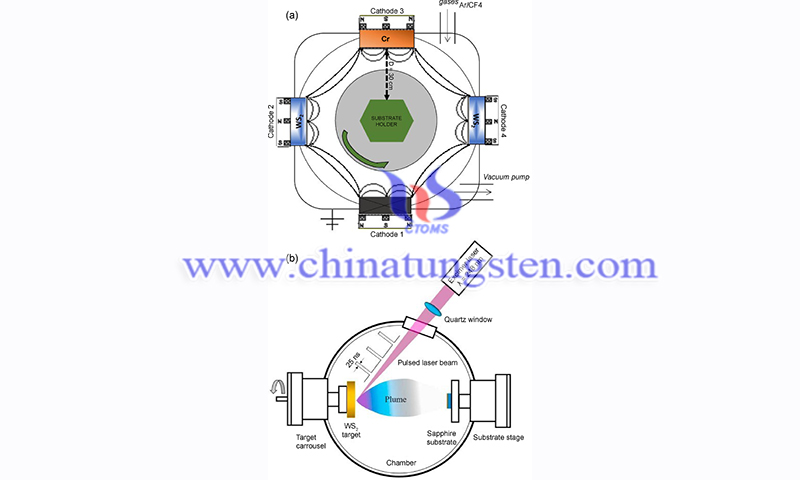
Owing to unique physical and chemical properties, transition metal dichalcogenides (TMDs) attract research interest. Among the family of TMDs, tungsten disulfide (WS2) has a unique band structure due to its semiconductor properties; i.e., its broadband spectral response characteristics, ultra-fast bleaching recovery time, and excellent saturable light absorption.
The 3 Most Common Tungsten Alloys—Its Properties & Applications
- Details
- Category: Tungsten Information
- Published on Tuesday, 06 September 2022 10:29
- Written by yuntao
- Hits: 1267

Alloys are metals made by combining two or more metallic elements, primarily to provide greater strength or corrosion resistance. The tungsten alloy family has many industrial applications due to its strength. Tungsten offers a unique contribution because it imparts exceptional strength, corrosion resistance and other useful properties to base metals. In addition to being an excellent alloying element, tungsten can also serve as the basis for its own alloys, and this article will focus on the basic categories of these tungsten alloys. Below are some details on the 3 most common tungsten alloys widely used in industry. Their properties and applications will also be introduced.
Read more: The 3 Most Common Tungsten Alloys—Its Properties & Applications
Atomic Structure of Tungsten Disulfide
- Details
- Category: Tungsten Information
- Published on Sunday, 11 September 2022 22:22
- Written by Caodan
- Hits: 1422
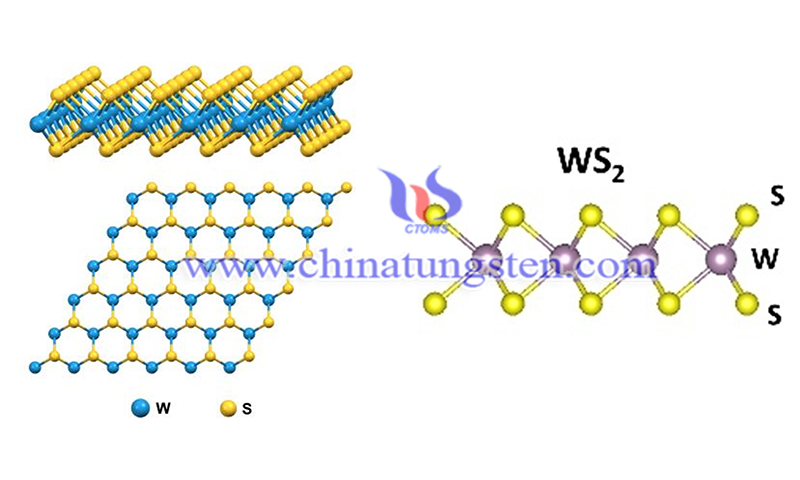
The atomic structure of tungsten disulfide (WS2) consists of a stack of three layers formed by a transition metal layer (W atom) sandwiched between two S-atom layers, each with a hexagonal lattice structure. In the three-layer stack, W and S atoms are bonded together by strong ion-covalent bonds. WS2 formed by these three layers is held together by weak van der Waals interactions, which allow mechanical exfoliation of the WS2 layer. In the bulk phase, polymorphism is a unique feature of TMDs.
Challenges of Current Applications on WS2 Nanomaterials
- Details
- Category: Tungsten Information
- Published on Monday, 05 September 2022 22:55
- Written by Caodan
- Hits: 1213

The current applications on WS2 nanomaterials still face challenges and should be investigated in depth in the following aspects. Firstly, the study mechanism of HER needs to be deepened and clarified in terms of the fundamental properties of WS2. Advanced characterization methods, such as in situ techniques, can be combined to analyze the structural changes of the material during the catalytic process and reveal the catalytic process of WS2-based nanomaterials, especially electrocatalysis.
Read more: Challenges of Current Applications on WS2 Nanomaterials





 sales@chinatungsten.com
sales@chinatungsten.com