Reasons of AMT / SiO2 The Catalyst Deactivation
- Details
- Category: Tungsten Information
- Published on Friday, 26 February 2016 18:18
SEM photograph of AMT / SiO2 catalyst before the reaction (fresh catalyst) and the deactivated catalyst after 615 h is shown in Figure 6. As can be seen, the fresh catalyst’s surface is clean and relatively homogeneous, the active species can be better uniformly distributed on the carrier; the catalyst surface is rough after deactivation, probably due to a large amount of coke on the catalyst surface coverage.
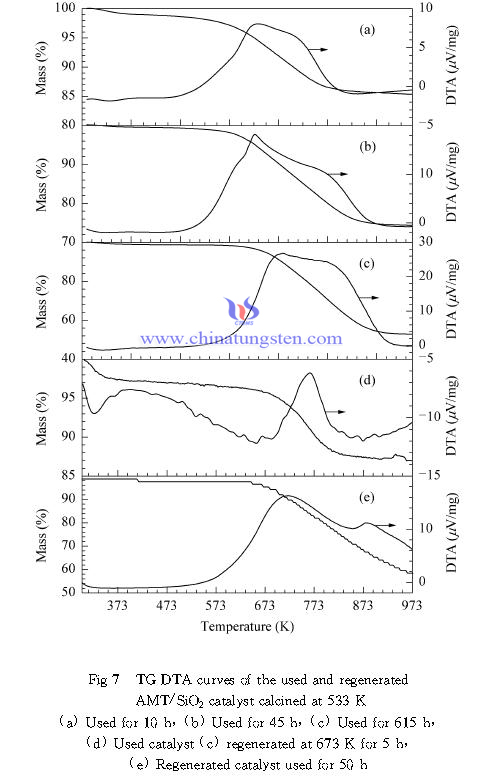
Figure 7 shows the TG-DTA spectrum of AMT / SiO2 catalyst calcined at 533K in different reaction time after calcination and regeneration. As can be seen, strong exothermic peak appears in the 50-900K range in all samples, and with the reaction time extending, the peak area and the catalyst weight loss was significantly increased. According to the preliminary work of catechol and ethanol monoetherification reaction on the surface of the multi-component phosphate catalyst coke behavioral findings, it is considered a low temperature exothermic peak (about 610K) mainly corresponds to adsorbed on the catalyst surface reactants and products, and exothermic peak temperature (650K or more) is mainly caused by the soot. Generally it believed that catechol and o-hydroxy anisole product can be further occurrence of oxygen and carbon alkylation alkylation reaction of carbon-containing polymer and ultimately the formation of coke, as the reaction time prolonged and temperature rise, carbon chain length of the polymer will gradually increase, which requires a higher firing temperatures to remove carbon. Therefore, we believe that prolonging the reaction time will significantly increase the amount of coke on the catalyst surface, which is the main reason for the catalyst deactivation.



| AMT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com