Tungsten Trioxide Electrochromic Mechanism
- Details
- Category: Tungsten Information
- Published on Wednesday, 27 January 2016 15:50
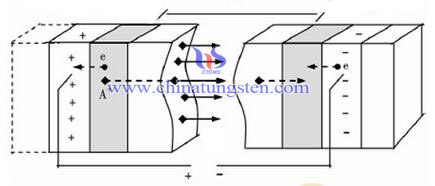 Electrochromic material has been able to achieve an electrochromic mainly in the chemical composition of the material band structure and redox properties. For example, electrons are injected and removing by changing the material of the additional electric field of ions, changing light absorption properties of the thin film, setting the thin film to light reflectance, but the detailed mechanism of various electrochromic materials has not been fully identified. Although Tungsten trioxide (WO3) is the first to be discovered photochromic material, but its electrochromic mechanism has been controversial. The experimental data and theoretical analysis models of Deb, Faughnan and Schirmer were established to explain the power of tungsten trioxide electrochromic mechanism.
Electrochromic material has been able to achieve an electrochromic mainly in the chemical composition of the material band structure and redox properties. For example, electrons are injected and removing by changing the material of the additional electric field of ions, changing light absorption properties of the thin film, setting the thin film to light reflectance, but the detailed mechanism of various electrochromic materials has not been fully identified. Although Tungsten trioxide (WO3) is the first to be discovered photochromic material, but its electrochromic mechanism has been controversial. The experimental data and theoretical analysis models of Deb, Faughnan and Schirmer were established to explain the power of tungsten trioxide electrochromic mechanism.
Deb model
The researchers developed a model that is the first to discover and produce tungsten trioxide thin film electrochromic device, also known as color center model. Deb made of amorphous WO3 study prepared by vacuum evaporation of the rule of law in 1973, the injected electrons are found in cathode form WO3. The oxygen vacancy defects capture F color centers, and ultimately proposed an amorphous WO3 ion crystal structure similar to metal halide (on the alkali halide crystals of the two electrodes voltage is applied and was heated at about 700 ℃. According to neutral and current continuity requirements, positive anion vacancies would cathode electrode near the movement in the positive electrode release halogen. This model explains the colored state WO3 thin film in oxygen to fade after high-temperature heating, the ability to disappear electrochromic phenomenon, the model proposed, but the opinion of Faughnan is that WO3-y film is large amount of oxygen deficiency (y = 0.5), it is difficult to produce large amounts of color centers.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com