Tungsten Trioxide Coulomb Titration Sulfur Effect
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 15:01
When tungsten trioxide in Coulomb titration burning sulfur, the organic sulfur can all break down at 800 ℃ ~ 900 ℃,while the sulphate sulfur requires to decompose at 1100 ℃ ~ 1200 ℃, so in order to ensure that coal various forms of sulfur can all decompose, there must be a higher oven temperature, on the other hand, SO2 + O2 = SO3-Q, between sulfur dioxide and sulfur trioxide in the presence of oxygen and heated environment, which is a reversible reaction, while trioxide sulfur does not participate in the reaction in the Coulomb titration process, which cause low determination conclusion .
We can see from the formula of sulfur dioxide and sulfur trioxide, this reaction is an exothermic reaction, the temperature rises will move toward the production of sulfur dioxide, so we should make the direction of the reaction to the reaction of sulfur dioxide and oxygen as far as possible In order to get accurate measurement results. The measurement results can be more accurate by the above two factors that are raising the temperature, but the temperature is too high will shorten the life of the combustion tube, thus, we have to reduce the combustion temperature, but must ensure that the sulfates completely decomposes at a lower temperature, so we cover a layer of catalyst on the coal.
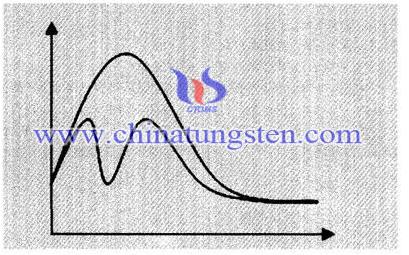 Tungsten Trioxide and Coal Principle During Combustion:
Tungsten Trioxide and Coal Principle During Combustion:
The catalyst is able to significantly change the reaction rate and the material, the chemical reaction itself, the chemical nature and quantity can be changed or not is according to the change of free energy, it can not determine the reaction occurs only according to the change of free energy, because chemical reaction also depends on the reaction energy barrier, that is, if the reaction energy barrier is high, we must provide the energy to fly over the energy barrier, the energy barrier known as the activation energy after completing the reaction, and the role of the catalyst is to reduce the activation of this reaction You can make it happen chemical reaction at relatively harsh environments.
The catalyst changes the reaction rate, which is due to changes in the reaction pathway, as the picture shows, by the reaction path requires a higher energy barrier is needed to change over the reaction pathway of low energy barrier, reducing the activation energy of the reaction. Tungsten trioxide plays a role of enabling sulphate complete decomposition in coal combustion process at a low temperature, which guarantees accurate measurement results.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com