Super Saturation Affects Ammonium Paratungstate Physical Properties 1/3
- Details
- Category: Tungsten Information
- Published on Wednesday, 30 December 2015 20:18
 Super saturation express as concentration driving force △c(△c=c-c0) [Ps: c for the super saturation concentration of the solution, known as concentration of main solution; c0 respective the saturated solubility concentration, means the equilibrium saturation concentration]. It considered that the crystal growth process of ammonium paratungstate is divided into three steps: non-basic element reaction, crystal medium diffusion and interface reaction. Instantaneous super saturation (△c) depends on supersaturated coming into being (polymerization) during the crystallization process and a D-value of eliminate (transformation). Linear growth rate related to △c(r1)and quantity growth rate (rm) can be used to reflect the relationship between them:
Super saturation express as concentration driving force △c(△c=c-c0) [Ps: c for the super saturation concentration of the solution, known as concentration of main solution; c0 respective the saturated solubility concentration, means the equilibrium saturation concentration]. It considered that the crystal growth process of ammonium paratungstate is divided into three steps: non-basic element reaction, crystal medium diffusion and interface reaction. Instantaneous super saturation (△c) depends on supersaturated coming into being (polymerization) during the crystallization process and a D-value of eliminate (transformation). Linear growth rate related to △c(r1)and quantity growth rate (rm) can be used to reflect the relationship between them:
r1=K1△c1, (K1means: line rate constant)
rm=Km s△c1, (km means :Quantity rate constant, s means: the existing grain surface area)
So, rm=kms2√ri/k1
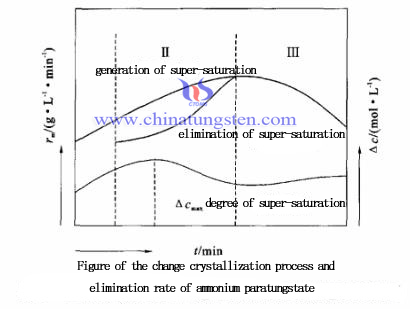
Three stages of the whole process of crystallization, the super saturation comes and the changing of elimination rate showed in the picture left:
From the figure, phaseⅠ: induction period, rm=0, the ammonium paratungstate crystallization process rate of super-saturation enlarged, △c1 continue to increasing. Phase II: crystal nucleus, rm>0, the rate of over saturation is always greater than that elimination, the phase transformation reaction to be the limit process of the crystallization. The early stage of the reaction due to the small s , △c still increasing, control by S phase. With the increase of S, △c reaches maximum value and began to decline, controlled by △c. Phase III: s keep increasing, the elimination rate of the super saturation equals to the generation, rm reach the maximum value. Value of rm decreases with the increasing rate of super-saturation, polymerization reaction is the limiting step in the crystallization process. Obviously, RM value of this stage can be used as a measure of the rate of super-saturation.
| APT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com