What Are the Chemical Properties of Tungsten Diiodide(I)?
- Details
- Category: Tungsten Information
- Published on Saturday, 09 September 2023 17:41
Tungsten diiodide (WI2) is an inorganic compound composed of the transition metal element tungsten and the halogen element iodine. It has many chemical properties, which will be described one by one below.
Oxidability
Tungsten diiodide has oxidizability and can undergo hydrogenation reaction with hydrogen at 500 ℃ to form metal tungsten. Macroscopically, it can be observed that the surface properties of reactants change from black powder solid to silvery white solid; Microscopically, two electrons in the valence orbital of tungsten lose their corresponding electrons in the combination with iodine, which makes the whole system produce a new equilibrium.
The reaction is theoretically deduced feasible analogy to iodine congeners fluorine and tungsten compounds tungsten hexafluoride is deduced to be the product of the reaction with hydrogen. Compared with tungsten hexafluoride, tungsten diiodide has weaker oxidizability, which means that it needs more stringent conditions to react.
The tungsten element of tungsten diiodide can also be reduced at high temperatures to produce a valence reaction: WI2 → W + I2 The temperature required for this reaction is extremely high, and this decomposition only exists at the filament (above 2000 ℃) in common iodine tungsten lamps.
Reducibility
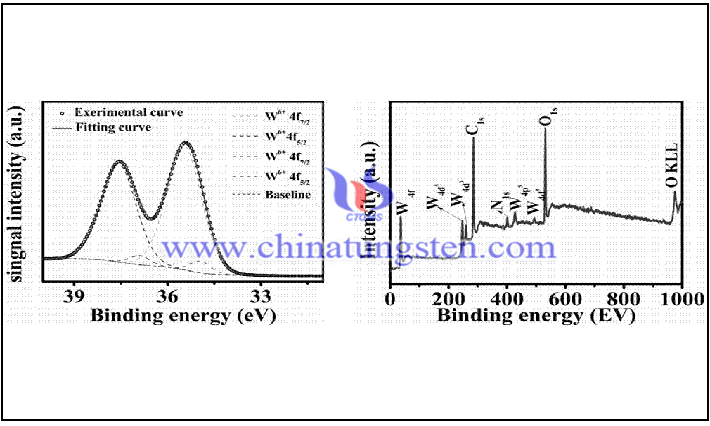
Tungsten diiodide is also reducible and can be heated and oxidized in air to form tungsten oxide. Moreover, it can be oxidized to more stable tungsten triiodide (WI3) or tungsten tetraiodide (WI4) depending on the reaction conditions and reactants. The reason why tungsten diiodide can be oxidized and reduced at the same time is that the valence state of tungsten in tungsten diiodide is +2. Observing the valence orbital of tungsten, we can find that there is one empty orbital, four lone pairs of electrons, and one pair of electrons. This means that tungsten can continue to increase its valence state and lose its valence state electrons when it loses two electrons. However, tungsten itself is chemically stable, and no matter what kind of reaction occurs, it needs to be promoted by the outside world.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com