What Is the Solubility of Tungsten Diiodide?
- Details
- Category: Tungsten Information
- Published on Wednesday, 14 June 2023 16:47
The solubility of tungsten diiodide has no specific pattern. For inorganic solvents, tungsten diiodide is soluble in alkali but insoluble in water at room temperature; for organic solvents, tungsten diiodide is insoluble in ethanol and carbon disulfide (CS2).
The solubility of tungsten diiodide is determined by many factors, structural similarity is in the first place. It is affected by the principle of similar solubility, which is an empirical rule about the solubility of substances. Generally speaking, the principle of similar solubility means that substances with similar structures are easily dissolved in each other, and the solubility is directly proportional to the structural similarity. In a narrow sense, it is equally soluble in a nonpolar or weakly polar solvent if a solute is the relevant molecule. Polarity here refers to the uneven charge distribution in the molecule. For example, the solvent carbon disulfide is a non-polar molecule, while the solute tungsten diiodide is not a non-polar molecule, so they are not mutually soluble.

The solubility of tungsten diiodide is also determined by inter-molecular forces. Tungsten iodide is not soluble in water at room temperature, but under heating conditions (80℃), it can react with water: WI2+2H2O→WO2↓+2HI+H2↑. According to the reaction, we can infer that tungsten iodide have changed the inter-molecular forces in the heating process, resulting in its similarity about the size and type of forces,which makes the two soluble and chemically react.
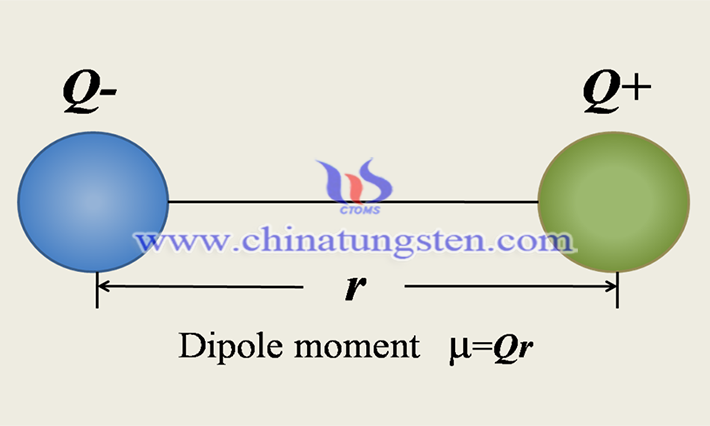
Additionally, the dipole distance between the solute and solvent molecules is also one of the factors affecting solubility. The dipole moment is defined as the product of the distance between the positive and negative charge centers of a molecule and the amount of electricity carried by the charge centers. The greater the value of the dipole moment, the greater the polarity of the bond. Although the dipole moment can quantify the polarity of covalent bonds numerically, it can not accurately reflect polarity of a molecule and needs to be analyzed in the structure and specific reaction.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com