Recent Developments in WS2 Energy Conversion and Storage Systems
- Details
- Category: Tungsten Information
- Published on Monday, 29 August 2022 21:54
WS2 has attracted much attention due to its unique structural properties and suitable hydrogen binding energy (comparable to platinum group metals). WS2 nanomaterials have been extensively investigated for energy conversion and storage systems.
Electrocatalytic HER is considered as a promising method to obtain H2, which relies on additional electrical energy to overcome the thermodynamic limit of HER. In HER, the catalyst is one of the most influential factors. Researchers have conducted numerous studies on electrocatalysts to find catalysts with efficient energy utilization and good reaction stability.
So far, platinum group metals have been used as the most effective electrocatalysts for HER applications in acidic media. however, their low reserves and high cost have largely hindered their large-scale application.
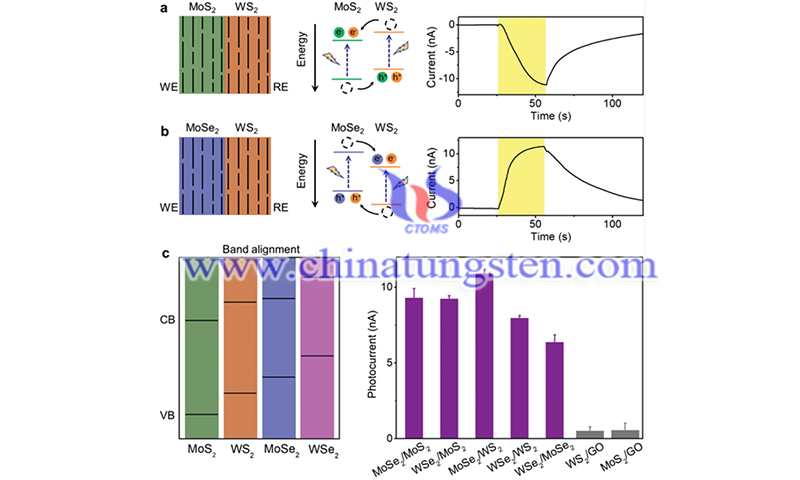
(Picture source: Jia P/Advanced Materials)
Therefore, the search for suitable substitutes that are abundant and inexpensive is an urgent task, which has stimulated various research. As an earth-abundant two-dimensional material, WS2 has received increasing attention due to its unique structural properties and suitable hydrogen binding energy. The electrocatalytic activity of WS2 depends largely on its surface structure and morphology. Large-scale fabrication of high-quality WS2 nanostructures with excellent catalytic activity is one of the issues under exploration.
To date, various morphologies have been investigated to improve the exposure of WS2 active sites. For example, Wu et al. fabricated WS2 nanosheets with loosely stacked layers that provide highly exposed active edges. The prepared WS2 showed an overpotential of -150 mV and a current density of ~9.66 mA.cm-2, which is much higher than the electrode of MoS2 (~1.12 Ma.cm-2).
In addition, Zou et al. demonstrated an effective and simple method to fabricate WS2 with three-dimensional nanoflower shapes. For effectively exposed active edges, the prepared materials exhibited good electrocatalytic properties. In addition, the researchers fabricated novel WS2 nanomaterials with highly exposed edges to the reactants, and thus, the prepared WS2@WS2 nanorattles exhibited outstanding stability and electrocatalytic activity.
However, the relatively low conductivity of WS2 (40-S·m-1) usually leads to high impedance. To face this challenge, many approaches have attempted to improve the conductivity and electrochemical properties of WS2, especially when compounded with carbon-based materials.
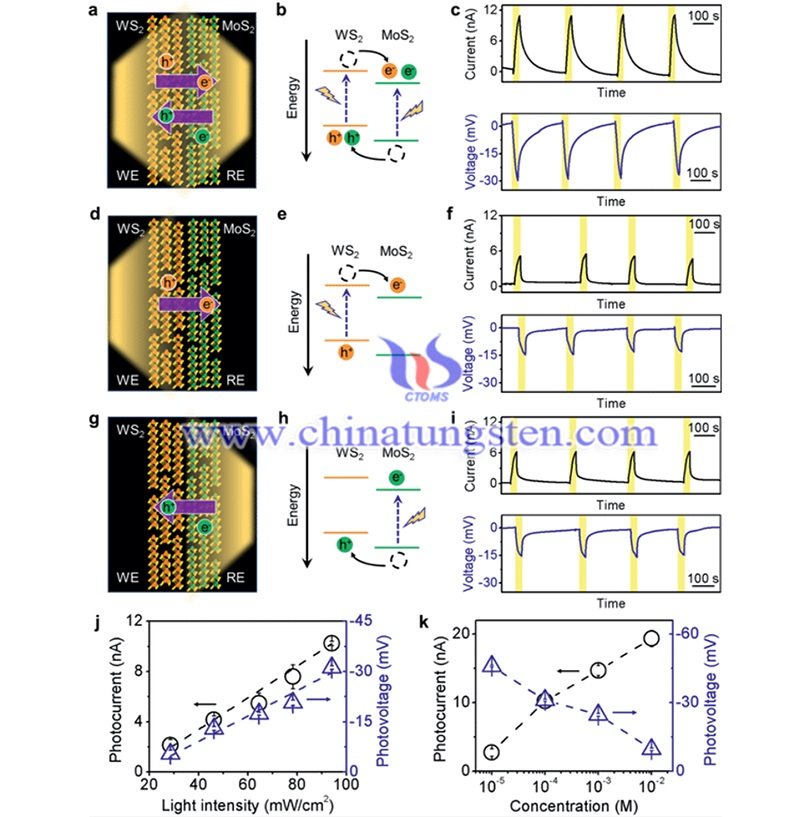
(Picture source: Jia P/Advanced Materials)
Electrochemical impedance spectroscopy (EIS) analysis showed that the WS2/RGO hybrid nanosheets possessed a low impedance system after annealing, and doping proved to be a feasible method to improve the electrocatalytic performance of WS2.
For example, Sun et al. prepared nitrogen-doped WS2 (N-WS2-Ar) under Ar gas and subsequent post-annealed samples in reduced H2 gas (N-WS2-H2). It was found that the nitrogen-doped WS2 monolayer exhibited a band gap of 1.5 eV, which is narrower than that of the bare WS2 monolayer (2.1 eV). More importantly, it is shown that dense hybridization of the p orbitals of the N atom and the d orbitals of the neighboring W atom, and the orbitals of the S atom at the Fermi level can excite more charge carriers, and thus, the intrinsic conductivity of the N-doped WS2 can be improved.
Another limitation is the adsorption and desorption process of H atoms. To facilitate the adsorption and desorption of H atoms, the researchers designed a WxC@WS2 nanostructure to enhance the evolutionary properties of hydrogen, revealing that the charge distribution between WxC and WS2 has an important effect on the simultaneous facilitation of the adsorption and desorption processes of H atoms.
Article Source: Sun, CB., Zhong, YW., Fu, WJ. et al. WS2 nanomaterials for energy conversion and storage. Tungsten 2, 109–133 (2020).
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com