Preparation of Sulfated WO3/SnO2 Catalysts Using APT
- Details
- Category: Tungsten Information
- Published on Friday, 25 June 2021 21:29
Many reactions in synthetic organic chemistry are acid-catalyzed reactions. These reactions include esterification, etherification, hydration, hydrolysis, alkylation, isomerization and others. Liquid-phase esterification is an important method for producing various esters. The esters are used in the manufacturing of solvents, plasticizers, plastics, leather, perfumes, and medicine.

Metal oxides, mixed oxides, cation exchange resins and zeolites were the most used solid acids. In the last decade, it is found that the loading of some metal oxides with sulfate ion or acidic high valence metal oxides such as WO3 and MoO3 causes an increase in the acidity of the catalyst and produces superacids.
Thus, sulfated WO3/SnO2 catalysts were prepared by using ammonium paratungstate (APT) as tungsten source, the synthesized catalysts have strong acid sites and contain both Brønsted and Lewis acid sites.The synthesis process of sulfated WO3/SnO2 catalysts is as below:
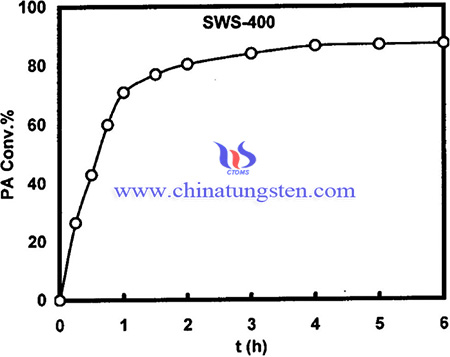
Pure tin oxide gel was prepared by a dropwise addition of ammonia solution (10 wt%) to 0.5 M solution of SnCl4.5H2O with vigorous stirring for 4 h, the final pH of the gel was adjusted to 8. The gel was left overnight washed by decantation with 1 wt% ammonium acetate solution until all chloride ions were eliminated (silver nitrate test), then washed with double distilled water and finally dried at 120 °C. Appropriate amount of ammonium paratungstate (APT) solution (30 g/L) (Prolabo) was added to the dry tin hydroxide gel, to obtain 15 wt%WO3 loading, with vigorous stirring for 4 h. The product was left overnight then dried at 120 °C. The WO3/SnO2 support was sulfated by the addition of an appropriate amount of 1 M H2SO4 solution, to obtain 15 wt%SO4 loading, with stirring for 4 h, followed by drying at 120 °C for 24 h. The prepared catalyst was calcined in air at 400, 500, 650 and 800 °C for 4 h.
In summary, XRD results reveal that WO3 dispersed completely on the surface of SnO2 and sulfating of WO3/SnO2 hinders the crystallization of SnO2. The textural properties are maxima at 500 °C. The catalysts possess very strong acid sites and contain both Brønsted and Lewis acid sites. The total surface acidity decreased with raising of the calcination temperature. The highest conversion of propionic acid was for 400 °C product, and decreased with an increase in calcination temperature. Sulfation enhances the surface acidity and increases the strength of Lewis acidity due to the inductive effect of Sdouble bondO.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com