New Modification Method to Enhance Red Phosphors with Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Sunday, 06 June 2021 02:31
White light-emitting diodes (LEDs) as a next generation light sources have attracted much attention due to their superior advantages over conventional incandescent and fluorescent lamps. Recently, numerous efforts have been focused to improve the color rendering index (CRI) of the white LEDs with the discovery of novel and efficient red emitting phosphor materials. Eu3+ activated luminescent materials such as sulphides, nitrides, phosphates, tungstates and molybdates were extensively investigated as red phosphors for white LEDs due to their high CRI values and low correlated color temperature over commercial YAG;Ce3+ based white LEDs.

The LGWM:Eu3+ red phosphors were produced by citric acid and ethylene glycol assisted sol-gel process. Ammonium paratungstate (APT) and ammonium molybdate tetrahydrate had been used to modify and enhance the red emitting ability of the designed red phosphors. The preparation method of the red phosphor is as following steps:
Lithium nitrate (LiNO3), gadolinium oxide (Gd2O3), europium oxide (Eu2O3), ammonium paratungstate (APT) and ammonium molybdate tetrahydrate were used as starting precursors. Citric acid and ethylene glycol were used as chelator and binder in the process respectively.
Firstly, calculated amounts of LiNO3, ammonium paratungstate and ammonium molybdate tetrahydrate were dissolved in deionized water separately. Gd(NO3)3 and Eu(NO3)3 solution was prepared by dissolving Gd2O3 and Eu2O3 with diluted nitric acid under stirring and heating to remove the excessive nitric acid. Then, citric acid was added separately with the clear metallic solution of nitrates and tungsto-molybdate. These metal citrates solution were mixed together and stirred vigorously with constant heating of 353 K for 20 min. The pH of the mixed solution was adjusted to 5.5 by adding ammonia solution. Ethylene glycol was added to promote the citrate polymerization and the ratio between metal ions, citric acid and ethylene glycol was kept as 0.9:1:1 in the present work. Rigid polyester net was formed, which prevents secondary phase segregation of metals during the polymer decomposition process. This mixture solution was dried under constant stirring and heating to obtain the polymer gel and pre-fired at 523 K. Further, the obtained powder was calcined at a temperature of 1073 K for 4 h and the resultant powders were characterized.
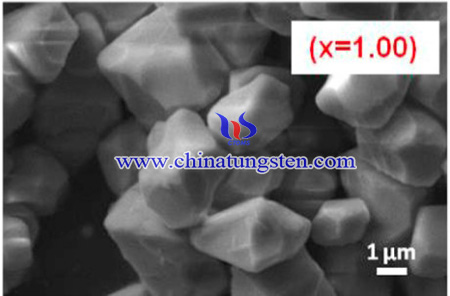
In summary, A series of LiGd(W1−xMoxO4)2:Eu3+ red emitting phosphors were successfully synthesized by sol-gel method. As the molybdate content increases, the particle morphology does not change and shows bulged sphere shape. Excitation spectrum indicate the broad and intense charge transfer band and the maximum intense red emission at 615 nm of 5D0→7F2 transition of Eu3+ ions when the relative ratio of Mo/W is 1:1. Therefore, these phosphors can be pumped using the near-UV (380–410 nm) and blue (~460 nm) LED chips to emit red light efficiently for various optoelectronic applications.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com