Crystalline Ammonium Tungsten Bronze by Thermal Decomposition of Ammonium Paratungstate (APT)
- Details
- Category: Tungsten Information
- Published on Sunday, 14 June 2020 00:29
The metastable phases of tungsten oxides or oxide bronzes, such as the hexagonal and the pyrochlore-types AxWO3, have caught much attention because of their one- or three-dimensional opened-tunneling structures. They have been used in the fields of electrochromic devices, humidity and gas sensors and secondary battery.
Tungsten bronzes are generally represented as AxWO3, though, they exist in different structural forms, and the color depths of them is controlled by the x value of the guest cation ‘A’.
The electronic conductivity of bronzes is due to the fact that charge compensation has to be made for the presence of A+ ions in the structure. This is achieved by changing the oxidation state of some tungsten atoms from the hexavalent to lower valencies. Thus, synthesis of tungsten bronzes have received a considerable attention.
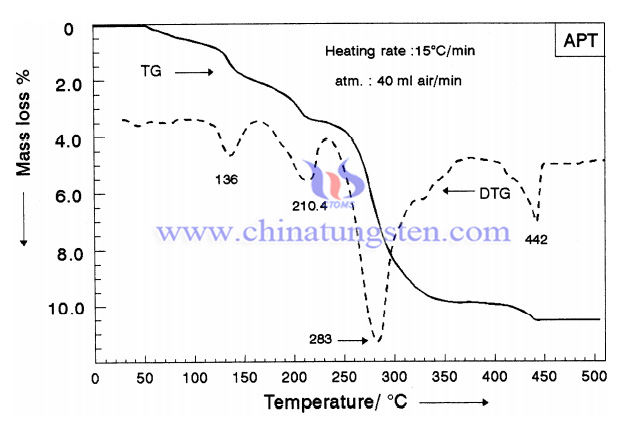
The conventional preparation of hexagonal ammonium tungsten bronze (NH4)0.33 WO3, is by hydrogen reduction of the ammonium tungstate (NH4)10W12O41·5H2O, at 350 °C. A thermal reduction of ammonium paratungstate (APT) to synthesis crystalline ammonium tungsten bronze has been conducted to deeply understand the process. The preparation steps are as follows:
The starting material, APT (99%) was heated at 400°C for 2 h in a fixed flow of air, then a yellowish powder is obtained. A particular amount of this powder was heated again for 2 h in in a fixed flow of air at 500°C, and a yellowish green product was obtained.
For comparison purposes, a pure WO3 (Degussa product, Germany) was obtained and subjected to the same physicochemical examination. X-ray diffractometry, infrared (IR), diffuse reflectance spectroscopy (DRS) and thermogravimetry (DTG) were used to analyse the products. The TG and DTG curves of the decomposition of ammonium paratungstate (APT) in a dynamic atmosphere (40 ml min−1) of air at a heating rate of 15°C min−1. TG and DTG curves were recorded on heating up to 600°C at 15°C min−1 in a stream of air (40 ml min−1).
In conclusion, thermal decomposition of APT generates a product containing crystalline ammonium tungsten bronze. The reducing atmosphere required is considered to be the NH3 a decomposition product of APT. Evidently, the thermal conditions applied enhance partial decomposition of the bronze into the more stable WO3 phase. Second thermal decomposition at 500°C, lead to thermal decomposition of ammonium tungsten bronze with liberation of ammonia and other decomposition products.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com