N−Doped WO3 Photocatalyst by Thermal Decomposition of Ammonium Paratungstate (APT)
- Details
- Category: Tungsten Information
- Published on Friday, 12 June 2020 06:19
Titanium dioxide (TiO2) might have wider applications in our daily life than you think. It has been applied in daily products, such as paints, toothpaste, food additive, dyes, sun creams, varnishes, textiles, paper and plastics. Another important application of TiO2 is as a semiconductor. It has the function to purify air, water and soil polluted with various hazardous chemicals.
Nonetheless, TiO2 photocatalysts has a shortage, where it requires a UV light irradiation wavelengths within 390 nm. In our previous reports, the visible light responsive TiO2 photocatalysts could be successfully prepared by using ion engineering techniques such as ion implantation or RF-magnetron sputtering deposition methods. Besides, the N-doping within the TiO2 semiconductor has recently been known to be effective for the preparation of the visible light responsive photocatalysts. In contrast, a tungsten trioxide (WO3) semiconductor, has been considered as a photocatalyst functioning under visible light irradiation. However, since the photo-excited electrons in the conduction band of WO3 semiconductor cannot reduce O2, it has been believed that photocatalytic oxidation of organic compounds hardly occurs on such bare WO3 surfaces. Nevertheless, WO3 loaded with Pt or CuO cocatalysts have recently been introduced to show the photocatalytic function under visible light irradiation.
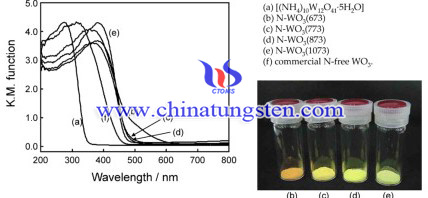
In this study, the N-doped WO3 semiconductor was prepared to expand its operation in visible light regions. The synthesis steps are as follows:
The N-doped WO3 powders were prepared by thermal decomposition of ammonium paratungstate (APT), at 473–1073 K. Small quantity of Pt particle was then loaded on the N-doped WO3 surface by a photodeposition method. The products were denoted as Pt(X)/N-WO3(Y) (X: loading amount of Pt (wt%), Y: calcination temperature (K)). A commercial N-free WO3 powder was used as a reference. The prepared N-WO3 samples were then characterized by XRD, diffuse reflectance UV–vis absorption (Shimadzu, UV-2200A) and XPS measurements at room temperature.
The photocatalytic reactivity of the Pt/N-WO3 samples was evaluated by decomposition of gaseous methanol under visible light or sunlight irradiations. The Pt/N-WO3 catalysts (50 mg) were placed onto a quartz cell (volume, ca. 33cm3).
The catalysts were degassed under high vacuum at 723 K for 2 h, treated in sufficient amounts of O2 at the same temperature for photocatalyst in the cell was cooled in a water bath. However, the water temperature increased up to 323–333 K during the photoreaction under sunlight irradiation. The amounts of CO2 produced in the photocatalytic reaction were then analyzed by a gas chromatography.
Conclusion
A novel N−doped WO3 semiconductor could be prepared by a simple calcination of an ammonium paratungstate containing NH+ ions. The NWO3 prepared at 673–873 K efficiently absorbed visible light in longer wavelength regions as compared to a commercial N-free WO3. The N-WO without Pt deposition did not show any photocatalytic reactivity. However, the Pt/N-WO3 samples showed much higher photocatalytic reactivity to decompose methanol of ca. 1000 ppm in gas phase under visible light having a wavelength longer than 450 nm or focused solar light irradiations. As well as, the Pt cocatalyst loaded on the N-WO3 surfaces was found to work as an efficient oxidation catalyst at low temperatures of 323–333 K. These results indicate that the newly prepared N-WO3 photocatalyst loaded with Pt cocatalyst can effectively utilize sunlight as light and/or heat sources.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com